
Corporate Presentation – Aug. 2018 Ibrexafungerp (formerly SCY-078) First Representative of a Novel Oral/IV Triterpenoid Antifungal Family “Committed to positively impacting the lives of patients suffering from difficult-to-treat and often life-threatening infections”
Forward-Looking Statements Certain statements regarding SCYNEXIS, Inc. (the “Company”) made in this presentation constitute forward-looking statements, including, but not limited to, statements regarding our business strategies and goals, plans and prospects, market size, adoption rate, potential revenue, clinical validity and utility, growth opportunities, future products and product pipeline. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from our expectations. These risks and uncertainties include, but are not limited, to: risks inherent in SCYNEXIS’s ability to successfully develop and obtain FDA approval for ibrexafungerp; the expected costs of studies and when they might begin or be concluded; and SCYNEXIS’s reliance on third parties to conduct SCYNEXIS’s clinical studies. Forward-looking statements may be identified by the use of the words “anticipates,” “expects,” “intends,” “plans,” “could,” “should,” “would,” “may,” “will,” “believes,” “estimates,” “potential,” or “continue” and variations or similar expressions. These statements are based upon the current expectations and beliefs of management and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include, but are not limited to, risks and uncertainties discussed in the Company's most recent reports filed with the Securities and Exchange Commission ("SEC"), including under the caption “Risks Factors” in the Company’s annual report on Form 10-K, which factors are incorporated herein by reference. Readers are cautioned not to place undue reliance on any of these forward-looking statements. The Company undertakes no obligation to update any of these forward-looking statements to reflect events or circumstances after the date of this presentation, or to reflect actual outcomes.
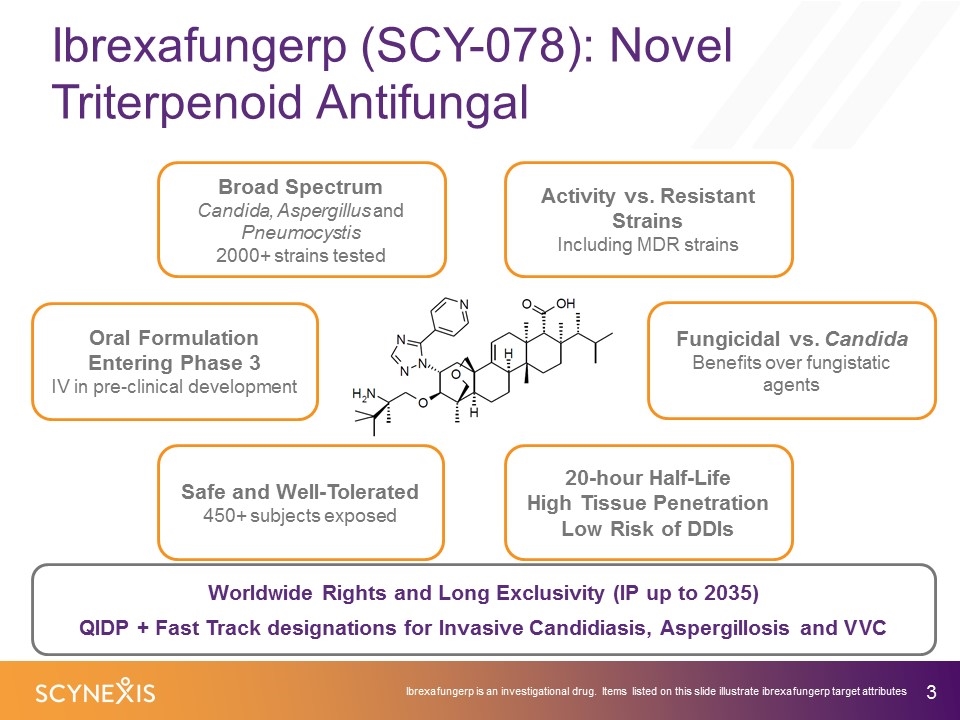
Ibrexafungerp (SCY-078): Novel Triterpenoid Antifungal Ibrexafungerp is an investigational drug. Items listed on this slide illustrate ibrexafungerp target attributes Safe and Well-Tolerated 450+ subjects exposed Oral Formulation Entering Phase 3 IV in pre-clinical development 20-hour Half-Life High Tissue Penetration Low Risk of DDIs Fungicidal vs. Candida Benefits over fungistatic agents Broad Spectrum Candida, Aspergillus and Pneumocystis 2000+ strains tested Worldwide Rights and Long Exclusivity (IP up to 2035) QIDP + Fast Track designations for Invasive Candidiasis, Aspergillosis and VVC Activity vs. Resistant Strains Including MDR strains
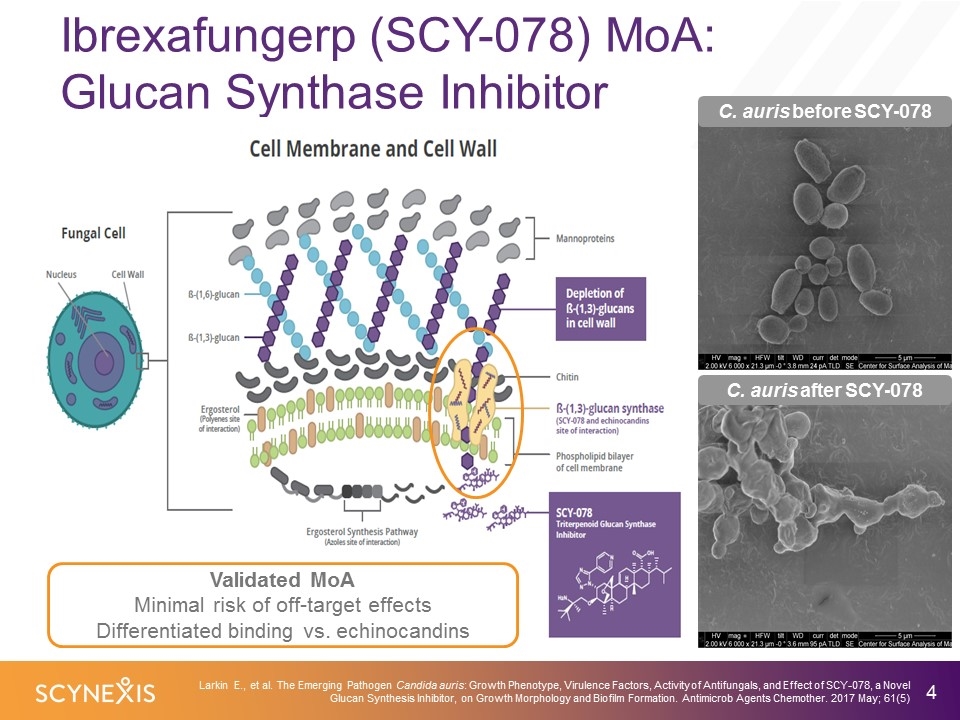
Ibrexafungerp (SCY-078) MoA: Glucan Synthase Inhibitor C. auris before SCY-078 C. auris after SCY-078 Larkin E., et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob Agents Chemother. 2017 May; 61(5) Validated MoA Minimal risk of off-target effects Differentiated binding vs. echinocandins
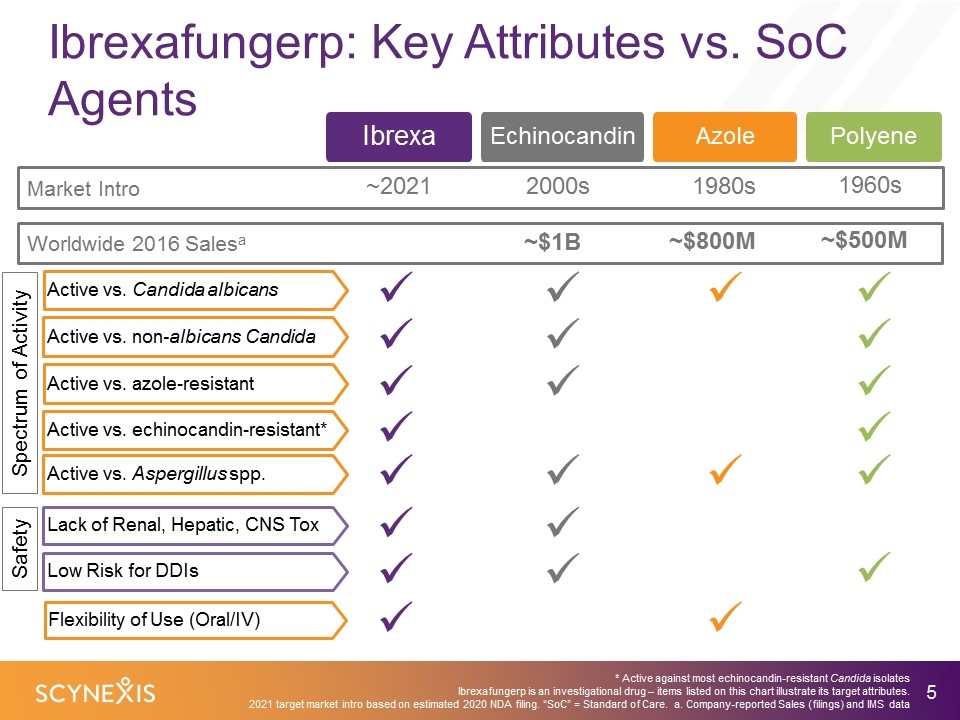
Ibrexafungerp: Key Attributes vs. SoC Agents ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü * Active against most echinocandin-resistant Candida isolates Ibrexafungerp is an investigational drug – items listed on this chart illustrate its target attributes. 2021 target market intro based on estimated 2020 NDA filing. “SoC” = Standard of Care. a. Company-reported Sales (filings) and IMS data Azole Ibrexa Echinocandin Market Intro ~2021 2000s 1980s 1960s Worldwide 2016 Salesa ~$1B ~$800M ~$500M Polyene Active vs. Candida albicans Active vs. non-albicans Candida Active vs. azole-resistant Flexibility of Use (Oral/IV) Active vs. echinocandin-resistant* Active vs. Aspergillus spp. Spectrum of Activity ü ü ü ü ü ü ü Lack of Renal, Hepatic, CNS Tox Low Risk for DDIs Safety ü
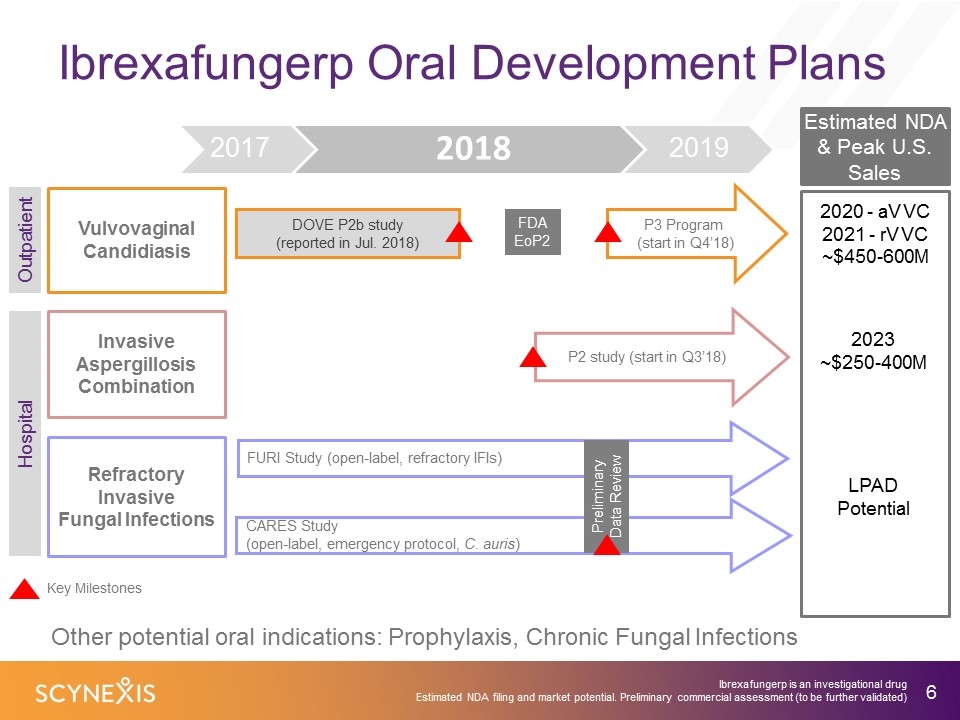
Ibrexafungerp Oral Development Plans 8 Vulvovaginal Candidiasis Invasive Aspergillosis Combination Refractory Invasive Fungal Infections CARES Study (open-label, emergency protocol, C. auris) P2 study (start in Q3’18) DOVE P2b study (reported in Jul. 2018) FURI Study (open-label, refractory IFIs) FDA EoP2 Preliminary Data Review P3 Program (start in Q4’18) Key Milestones Estimated NDA & Peak U.S. Sales 2020 - aVVC 2021 - rVVC ~$450-600M 2023 ~$250-400M LPAD Potential Ibrexafungerp is an investigational drug Estimated NDA filing and market potential. Preliminary commercial assessment (to be further validated) Other potential oral indications: Prophylaxis, Chronic Fungal Infections Outpatient Hospital 2017 2019 2018
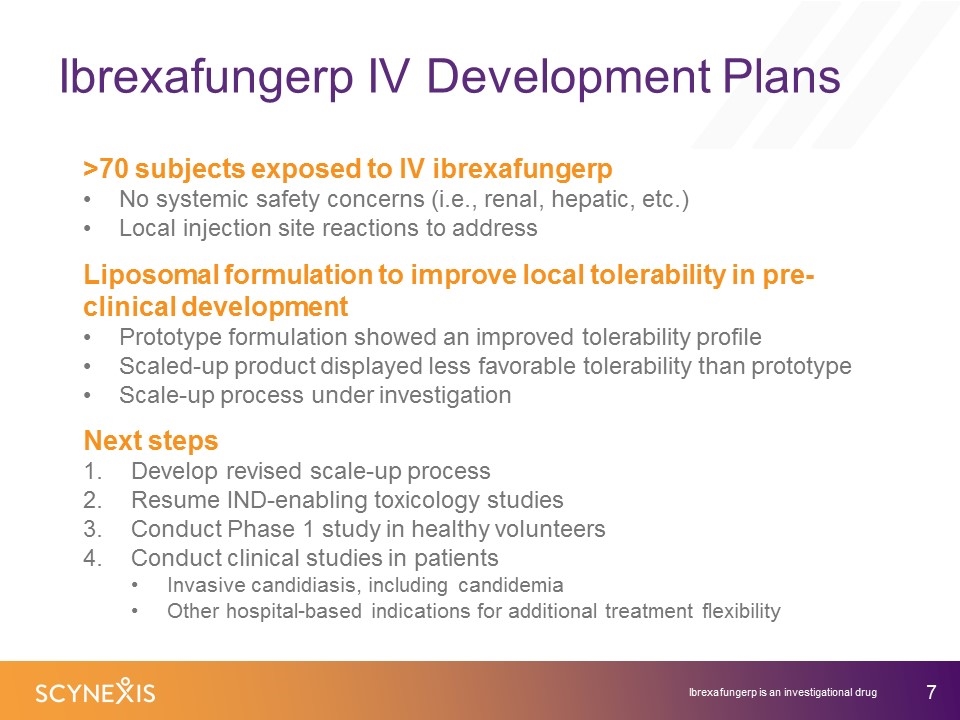
Ibrexafungerp IV Development Plans Ibrexafungerp is an investigational drug >70 subjects exposed to IV ibrexafungerp No systemic safety concerns (i.e., renal, hepatic, etc.) Local injection site reactions to address Liposomal formulation to improve local tolerability in pre-clinical development Prototype formulation showed an improved tolerability profile Scaled-up product displayed less favorable tolerability than prototype Scale-up process under investigation Next steps Develop revised scale-up process Resume IND-enabling toxicology studies Conduct Phase 1 study in healthy volunteers Conduct clinical studies in patients Invasive candidiasis, including candidemia Other hospital-based indications for additional treatment flexibility

Vulvovaginal Candidiasis (VVC) “Many of the unresolved clinical issues in managing women with rVVC would disappear if truly fungicidal drugs and regimens were available.” Dr. Jack Sobel Curr. Infect. Dis. Rep.2006,8:481–486
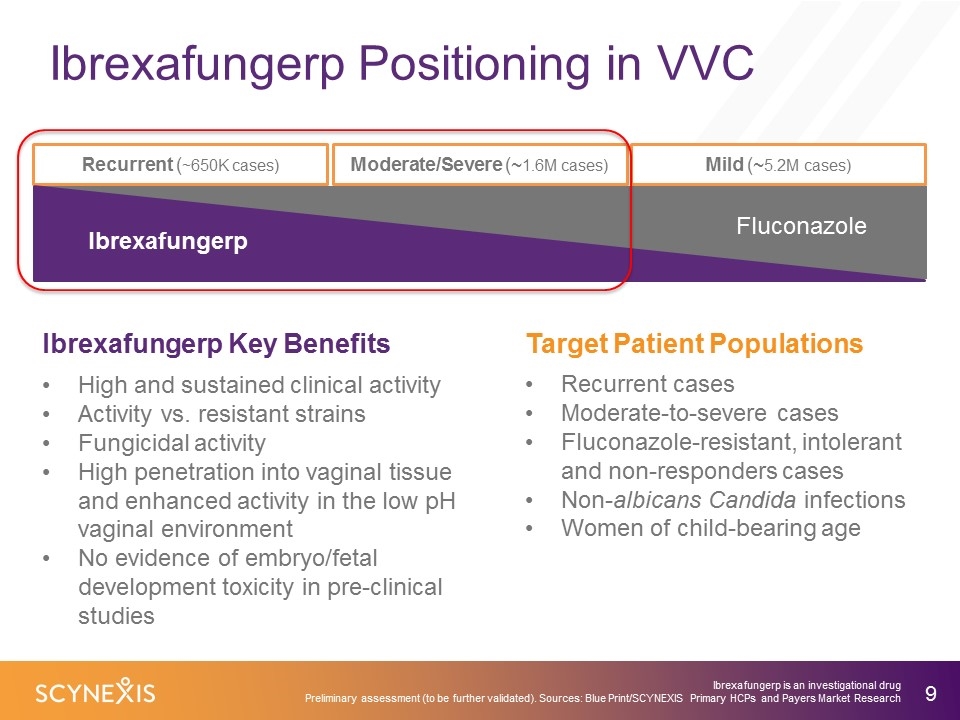
Ibrexafungerp Positioning in VVC Ibrexafungerp is an investigational drug Preliminary assessment (to be further validated). Sources: Blue Print/SCYNEXIS Primary HCPs and Payers Market Research Moderate/Severe (~1.6M cases) Mild (~5.2M cases) Recurrent (~650K cases) Fluconazole Ibrexafungerp High and sustained clinical activity Activity vs. resistant strains Fungicidal activity High penetration into vaginal tissue and enhanced activity in the low pH vaginal environment No evidence of embryo/fetal development toxicity in pre-clinical studies Ibrexafungerp Key Benefits Target Patient Populations Recurrent cases Moderate-to-severe cases Fluconazole-resistant, intolerant and non-responders cases Non-albicans Candida infections Women of child-bearing age
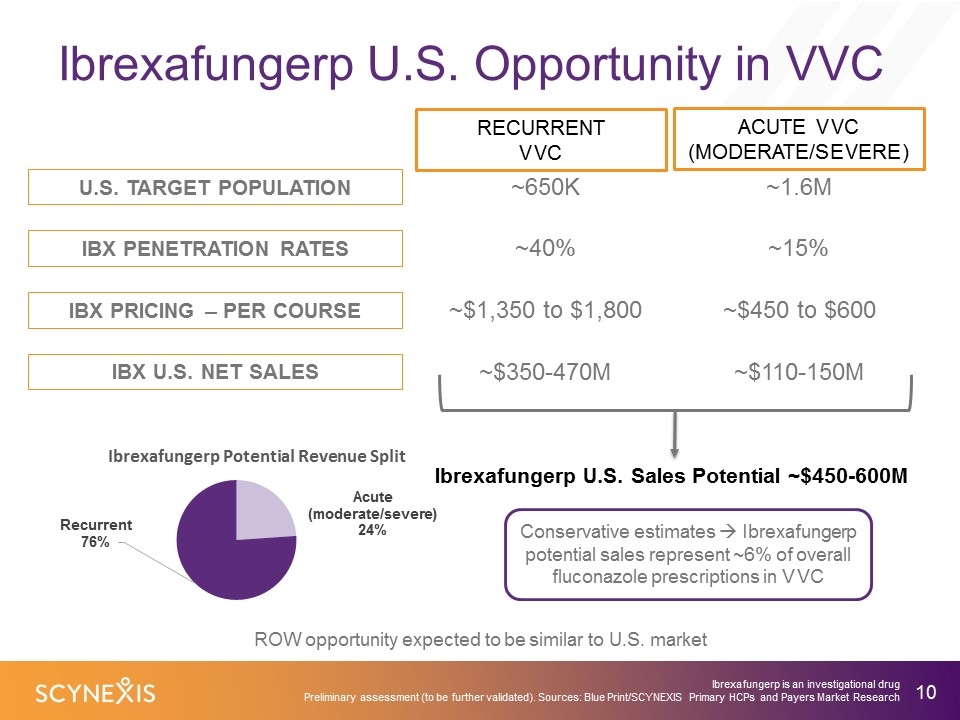
Ibrexafungerp U.S. Opportunity in VVC Ibrexafungerp U.S. Sales Potential ~$450-600M Conservative estimates à Ibrexafungerp potential sales represent ~6% of overall fluconazole prescriptions in VVC ROW opportunity expected to be similar to U.S. market U.S. TARGET POPULATION IBX PENETRATION RATES IBX PRICING – PER COURSE IBX U.S. NET SALES ACUTE VVC (MODERATE/SEVERE) ~1.6M ~650K ~15% ~40% ~$450 to $600 ~$1,350 to $1,800 ~$110-150M ~$350-470M Ibrexafungerp is an investigational drug Preliminary assessment (to be further validated). Sources: Blue Print/SCYNEXIS Primary HCPs and Payers Market Research RECURRENT VVC
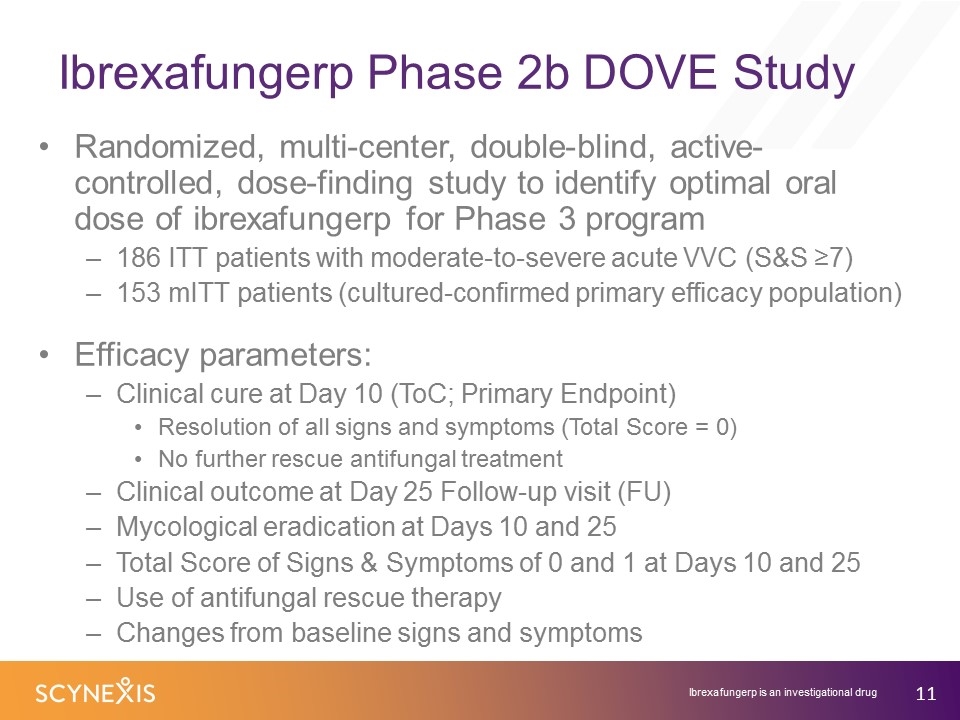
Ibrexafungerp Phase 2b DOVE Study Randomized, multi-center, double-blind, active-controlled, dose-finding study to identify optimal oral dose of ibrexafungerp for Phase 3 program 186 ITT patients with moderate-to-severe acute VVC (S&S ≥7) 153 mITT patients (cultured-confirmed primary efficacy population) Efficacy parameters: Clinical cure at Day 10 (ToC; Primary Endpoint) Resolution of all signs and symptoms (Total Score = 0) No further rescue antifungal treatment Clinical outcome at Day 25 Follow-up visit (FU) Mycological eradication at Days 10 and 25 Total Score of Signs & Symptoms of 0 and 1 at Days 10 and 25 Use of antifungal rescue therapy Changes from baseline signs and symptoms Ibrexafungerp is an investigational drug
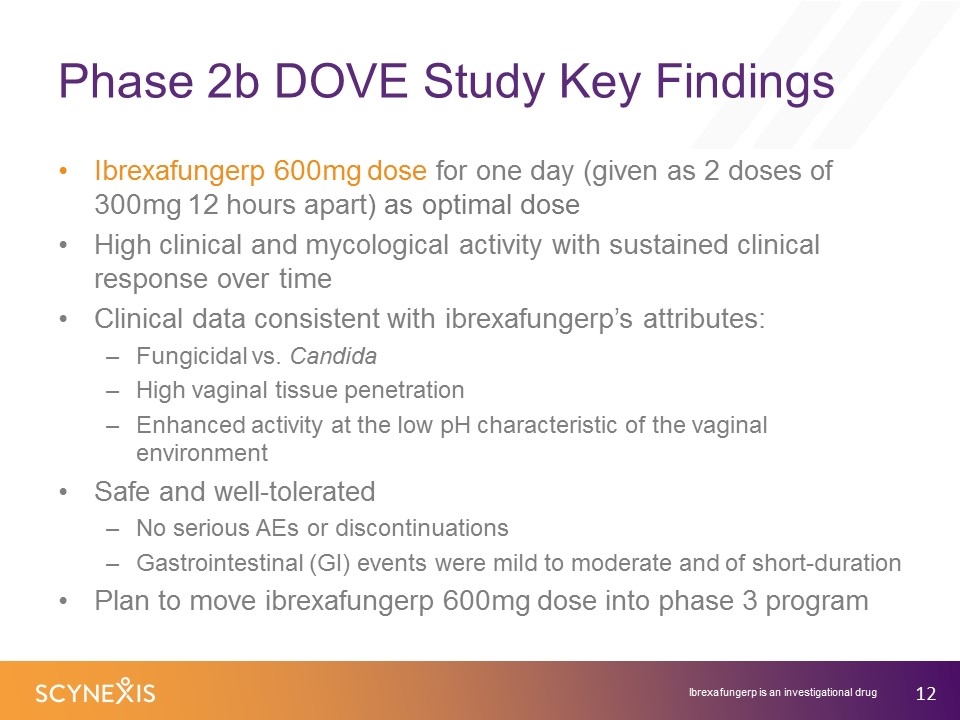
Phase 2b DOVE Study Key Findings Ibrexafungerp 600mg dose for one day (given as 2 doses of 300mg 12 hours apart) as optimal dose High clinical and mycological activity with sustained clinical response over time Clinical data consistent with ibrexafungerp’s attributes: Fungicidal vs. Candida High vaginal tissue penetration Enhanced activity at the low pH characteristic of the vaginal environment Safe and well-tolerated No serious AEs or discontinuations Gastrointestinal (GI) events were mild to moderate and of short-duration Plan to move ibrexafungerp 600mg dose into phase 3 program Ibrexafungerp is an investigational drug
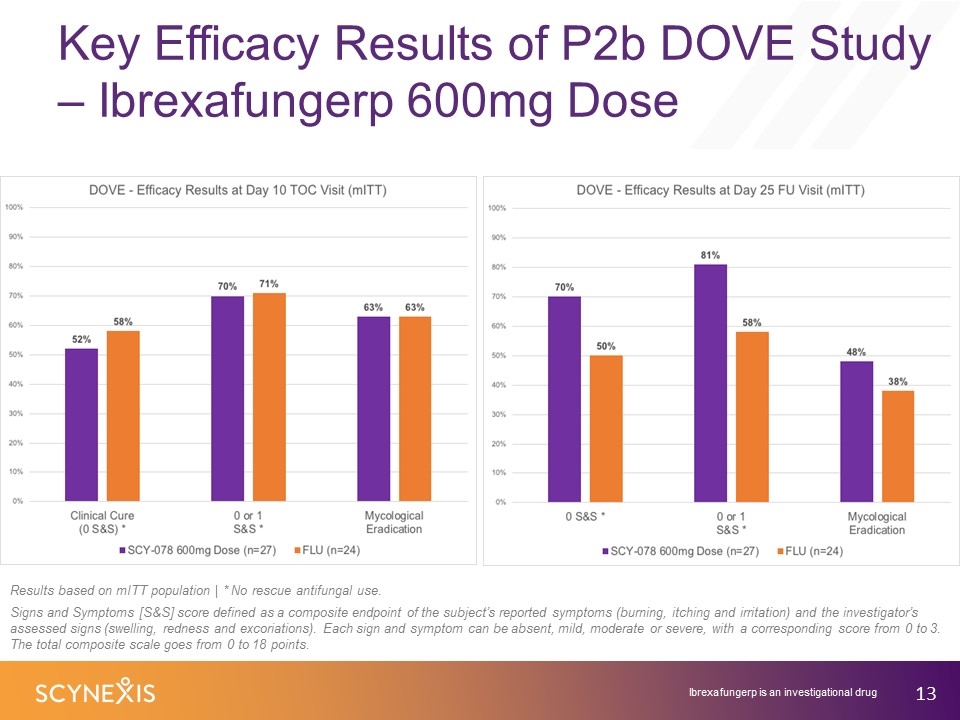
Key Efficacy Results of P2b DOVE Study – Ibrexafungerp 600mg Dose Results based on mITT population | * No rescue antifungal use. Signs and Symptoms [S&S] score defined as a composite endpoint of the subject’s reported symptoms (burning, itching and irritation) and the investigator’s assessed signs (swelling, redness and excoriations). Each sign and symptom can be absent, mild, moderate or severe, with a corresponding score from 0 to 3. The total composite scale goes from 0 to 18 points. Ibrexafungerp is an investigational drug
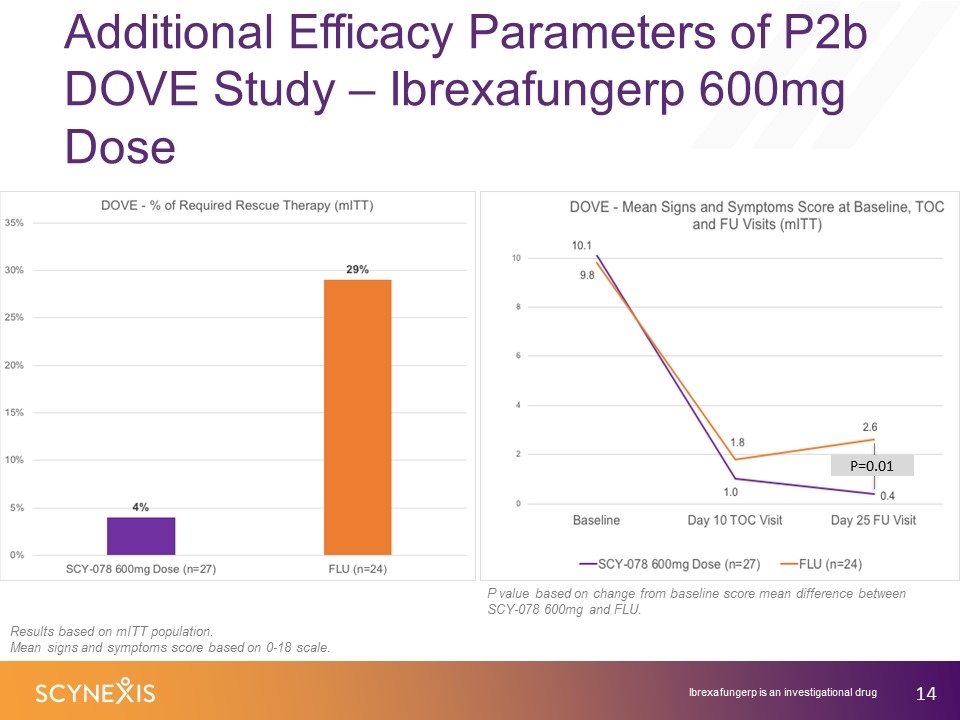
Additional Efficacy Parameters of P2b DOVE Study – Ibrexafungerp 600mg Dose Results based on mITT population. Mean signs and symptoms score based on 0-18 scale. P value based on change from baseline score mean difference between SCY-078 600mg and FLU. P=0.01 Ibrexafungerp is an investigational drug
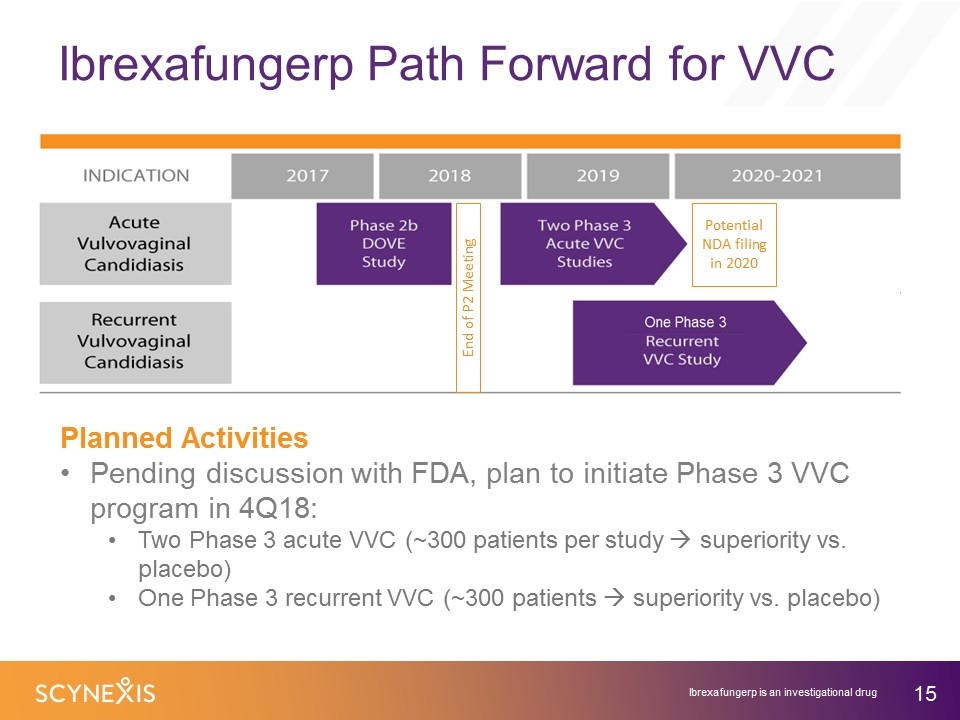
Ibrexafungerp Path Forward for VVC Planned Activities Pending discussion with FDA, plan to initiate Phase 3 VVC program in 4Q18: Two Phase 3 acute VVC (~300 patients per study à superiority vs. placebo) One Phase 3 recurrent VVC (~300 patients à superiority vs. placebo) End of P2 Meeting Potential NDA filing in 2020 Ibrexafungerp is an investigational drug

Invasive Aspergillosis (IA) “Invasive fungal infections will not go away any time soon. Therefore, we need to circumvent resistance to treatment by continued discovery and development of new antifungal agents and strategies.” Dr. John Perfect Nature Reviews/Drug Discoveries (2017)
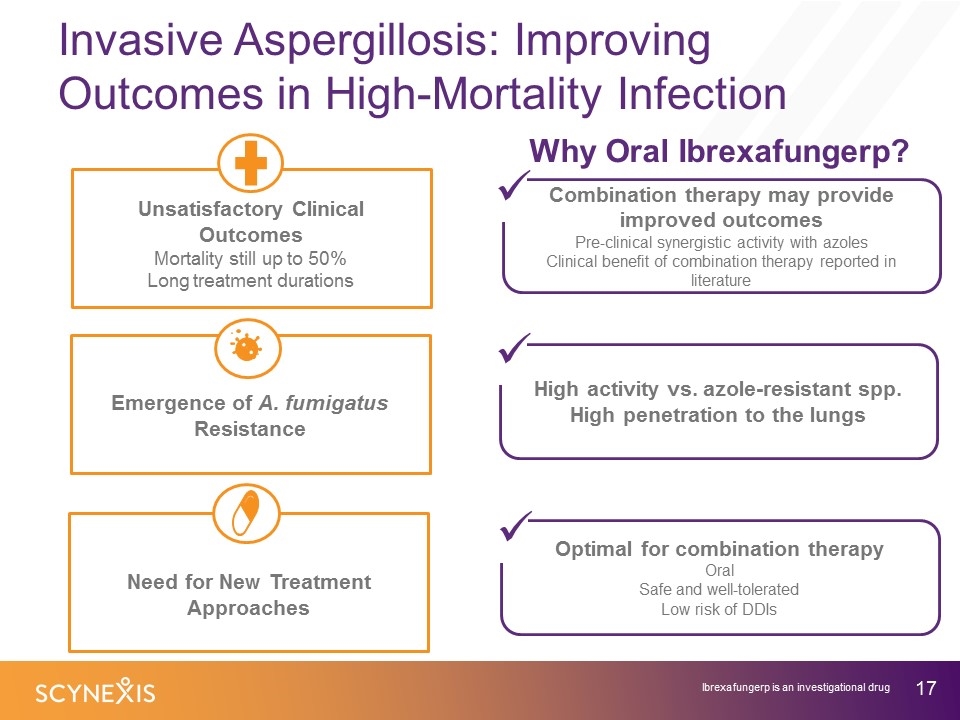
Invasive Aspergillosis: Improving Outcomes in High-Mortality Infection Why Oral Ibrexafungerp? High activity vs. azole-resistant spp. High penetration to the lungs Optimal for combination therapy Oral Safe and well-tolerated Low risk of DDIs Emergence of A. fumigatus Resistance Need for New Treatment Approaches Unsatisfactory Clinical Outcomes Mortality still up to 50% Long treatment durations Combination therapy may provide improved outcomes Pre-clinical synergistic activity with azoles Clinical benefit of combination therapy reported in literature ü Ibrexafungerp is an investigational drug ü ü
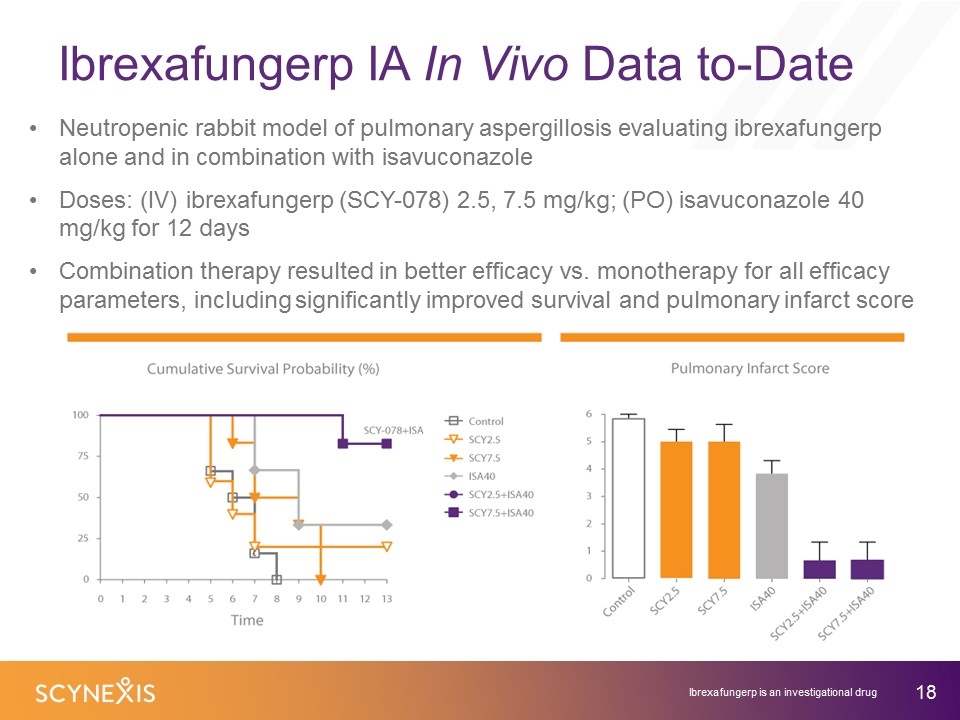
Ibrexafungerp IA In Vivo Data to-Date Neutropenic rabbit model of pulmonary aspergillosis evaluating ibrexafungerp alone and in combination with isavuconazole Doses: (IV) ibrexafungerp (SCY-078) 2.5, 7.5 mg/kg; (PO) isavuconazole 40 mg/kg for 12 days Combination therapy resulted in better efficacy vs. monotherapy for all efficacy parameters, including significantly improved survival and pulmonary infarct score Ibrexafungerp is an investigational drug
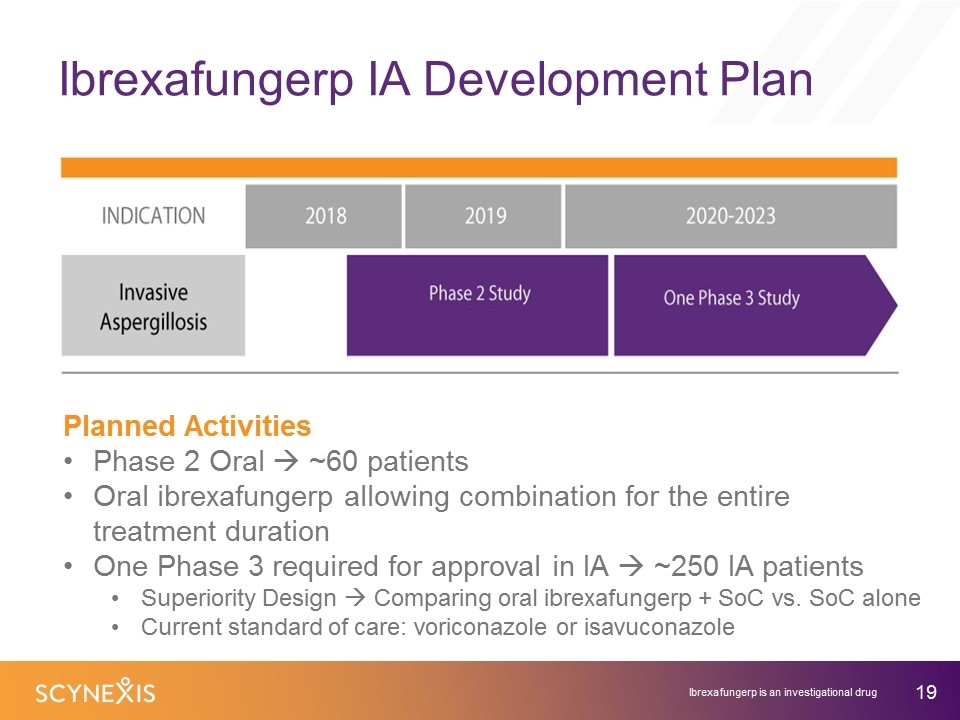
Ibrexafungerp IA Development Plan Ibrexafungerp is an investigational drug Planned Activities Phase 2 Oral à ~60 patients Oral ibrexafungerp allowing combination for the entire treatment duration One Phase 3 required for approval in IA à ~250 IA patients Superiority Design à Comparing oral ibrexafungerp + SoC vs. SoC alone Current standard of care: voriconazole or isavuconazole

Refractory Invasive Fungal Infections (rIFI) “Invasive fungal infections will not go away any time soon. Therefore, we need to circumvent resistance to treatment by continued discovery and development of new antifungal agents and strategies.” Dr. John Perfect Nature Reviews/Drug Discoveries (2017)
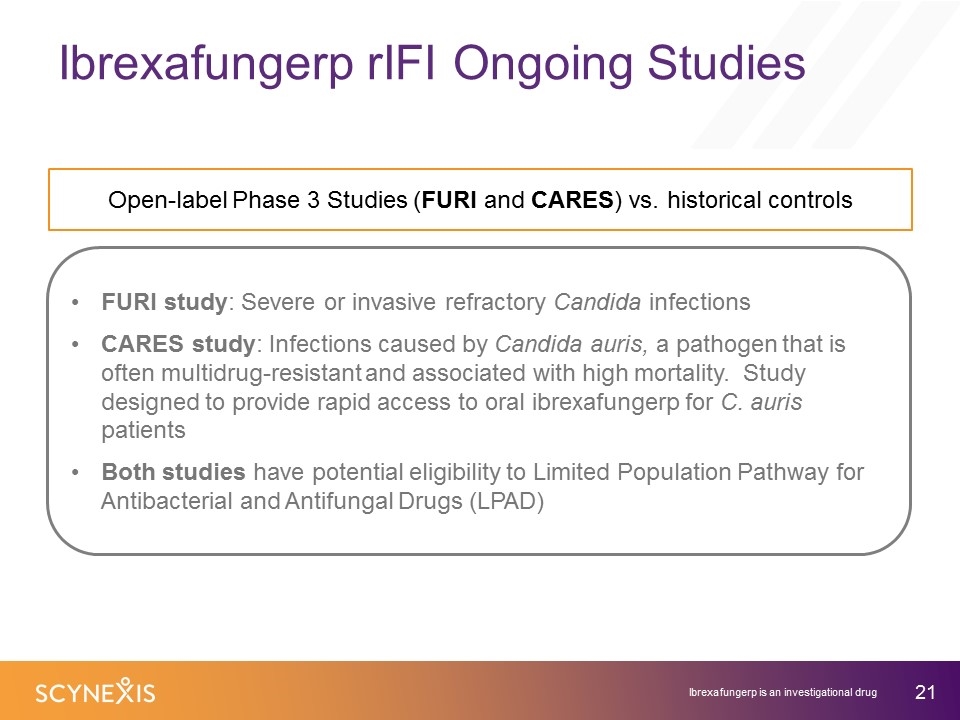
Ibrexafungerp rIFI Ongoing Studies Open-label Phase 3 Studies (FURI and CARES) vs. historical controls FURI study: Severe or invasive refractory Candida infections CARES study: Infections caused by Candida auris, a pathogen that is often multidrug-resistant and associated with high mortality. Study designed to provide rapid access to oral ibrexafungerp for C. auris patients Both studies have potential eligibility to Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) Ibrexafungerp is an investigational drug

SCYX: Experienced Management Positive track record in drug development & antifungal expertise CEO: Marco Taglietti, M.D. Schering-Plough, Stiefel, Forest Labs CMO: David Angulo, M.D. Schering-Plough, Stiefel, Brickell Biotech CFO: Eric Francois Cowen, Lazard, Topi General Counsel: Scott Sukenick Cooley Diverse backgrounds & operating experience in healthcare Guy Macdonald, Chairman (Tetraphase, Merck) Steven Gilman, PhD (Contrafect, Cubist) Ann Hanham, PhD (BAR Capital, Burrill, FDA) David Hastings (Arbutus Biopharma,Unilife, Incyte) Patrick Machado (Medivation) Marion McCourt (Regeneron, Medivation, Amgen) Leadership Board of Directors
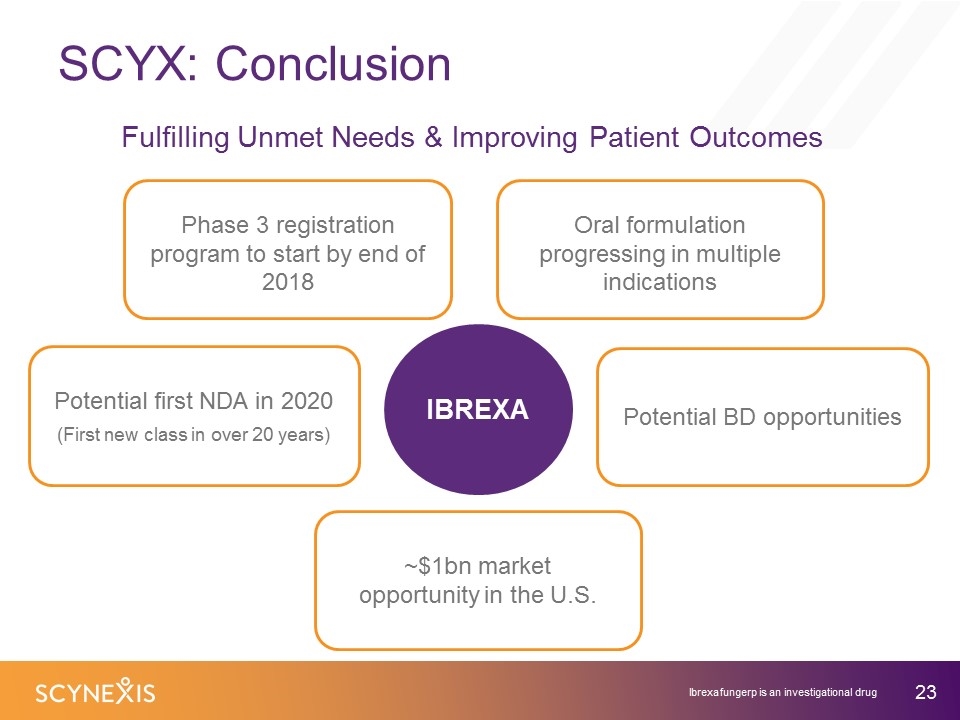
SCYX: Conclusion ~$1bn market opportunity in the U.S. Potential BD opportunities IBREXA Potential first NDA in 2020 (First new class in over 20 years) Phase 3 registration program to start by end of 2018 Fulfilling Unmet Needs & Improving Patient Outcomes Oral formulation progressing in multiple indications Ibrexafungerp is an investigational drug