

SCY-078 – First Representative of a Novel Oral/IV Triterpenoid Antifungal Family CORPORATE PRESENTATION | Mar. 2018

Certain statements regarding SCYNEXIS, Inc. (the “Company”) made in this presentation constitute forward-looking statements, including, but not limited to, statements regarding our business strategies and goals, plans and prospects, market size, adoption rate, potential revenue, clinical validity and utility, growth opportunities, future products and product pipeline. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from our expectations. These risks and uncertainties include, but are not limited, to: risks inherent in SCYNEXIS' ability to successfully develop SCY-078, to obtain FDA approval for SCY-078; the expected costs of studies and when they might begin or be concluded; and SCYNEXIS' reliance on third parties to conduct SCYNEXIS' clinical studies. Forward-looking statements may be identified by the use of the words “anticipates,” “expects,” “intends,” “plans,” “could,” “should,” “would,” “may,” “will,” “believes,” “estimates,” “potential,” or “continue” and variations or similar expressions. These statements are based upon the current expectations and beliefs of management and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include, but are not limited to, risks and uncertainties discussed in the Company's most recent reports filed with the Securities and Exchange Commission ("SEC") including under the caption “Risks Factors” in the Company’s annual report on Form 10-K, which factors are incorporated herein by reference. Readers are cautioned not to place undue reliance on any of these forward-looking statements. The Company undertakes no obligation to update any of these forward-looking statements to reflect events or circumstances after the date of this presentation, or to reflect actual outcomes. Forward-Looking Statements

General Information SCY-078 – First of a Novel Oral/IV Triterpenoid Antifungal Family Committed to positively impacting the lives of patients suffering from difficult-to-treat and often life-threatening infections
Positive track record in drug development & antifungal expertise Diverse backgrounds & operating experience in healthcare Experienced Management Team and Board SCYX: Investment Opportunity SCY-078 is an investigational drug. Items listed on this slide illustrate SCY-078 target attributes. SCY-078: Novel, first-in-class, IV/Oral Antifungal Versatile platform/multiple indications = further de-risked asset Late-Stage Product with Platform of Indications SCY-078: $1B+ market opportunity in the U.S. Potential first NDA in 2020 Attractive Market Opportunity Phase 2 data in mid-year Multiple clinical study initiations in second half New IV formulation in humans by Q3 Multiple Key Milestones in 2018
SCY-078: A Novel Triterpenoid Antifungal SCY-078 is an investigational drug. Items listed on this slide illustrate SCY-078 target attributes. * Including pre-clinical and clinical trial results. Flexible Dosing IV / Oral Multiple indications possible Safe and Well-Tolerated 400+ subjects exposed High Tissue Penetration Multiple in vivo models 20-hour t½ allowing QD Use Low Risk of DDIs 14 Phase 1 studies Validated MoA Minimal off-target effects Fungicidal vs. Candida High success rate in VVC Broad Spectrum (incl. MDR Strains) 2,000+ strains tested Worldwide Rights and Long Exclusivity (IP up to 2035) QIDP, Fast Track and Orphan Drug status for Invasive Candidiasis and Aspergillosis Product attributes supported by strong scientific evidence*
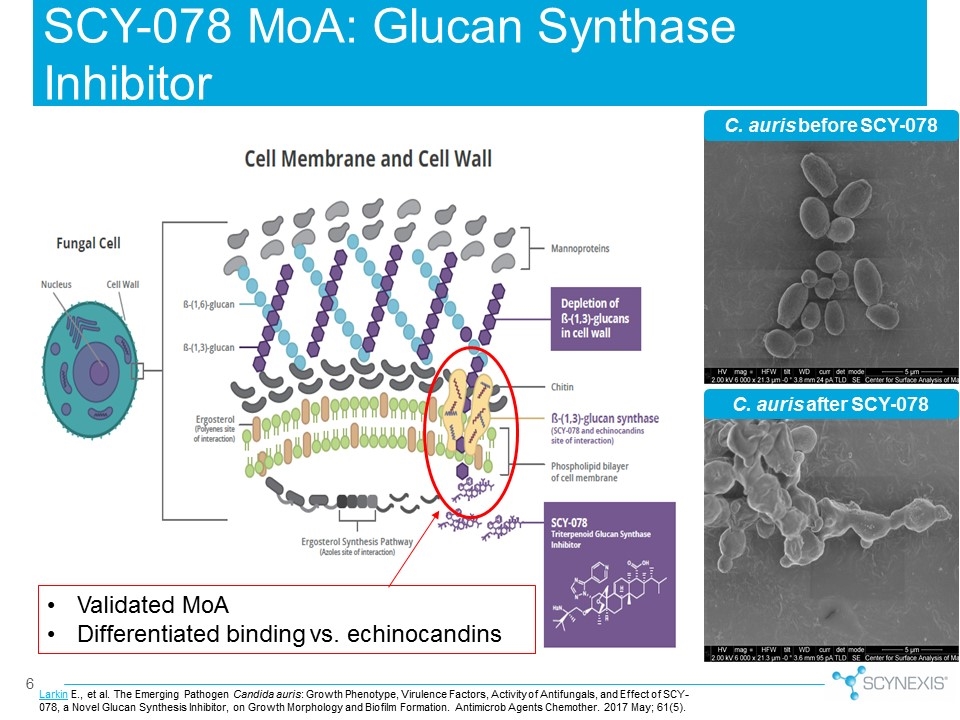
C. auris before SCY-078 C. auris after SCY-078 Larkin E., et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob Agents Chemother. 2017 May; 61(5). SCY-078 MoA: Glucan Synthase Inhibitor Validated MoA Differentiated binding vs. echinocandins
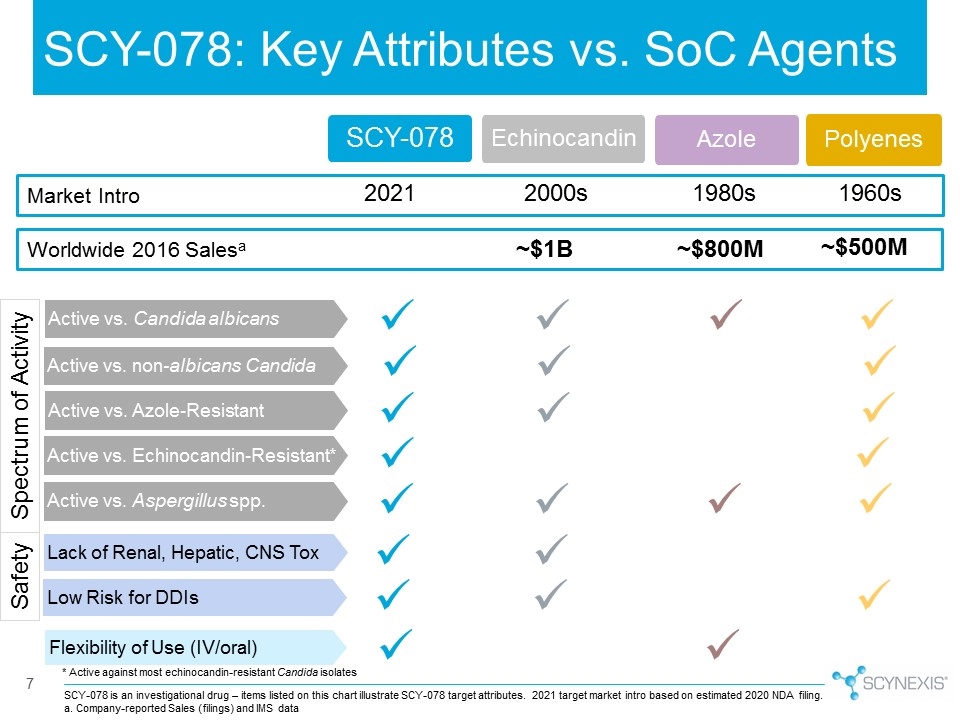
SCY-078 is an investigational drug – items listed on this chart illustrate SCY-078 target attributes. 2021 target market intro based on estimated 2020 NDA filing. a. Company-reported Sales (filings) and IMS data Azole SCY-078 7 Echinocandin SCY-078: Key Attributes vs. SoC Agents * Active against most echinocandin-resistant Candida isolates Market Intro 2021 2000s 1980s 1960s Worldwide 2016 Salesa ~$1B ~$800M ~$500M Polyenes Active vs. Candida albicans Active vs. non-albicans Candida Active vs. Azole-Resistant Flexibility of Use (IV/oral) Active vs. Echinocandin-Resistant* Active vs. Aspergillus spp. Spectrum of Activity ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü Lack of Renal, Hepatic, CNS Tox Low Risk for DDIs Safety ü ü ü
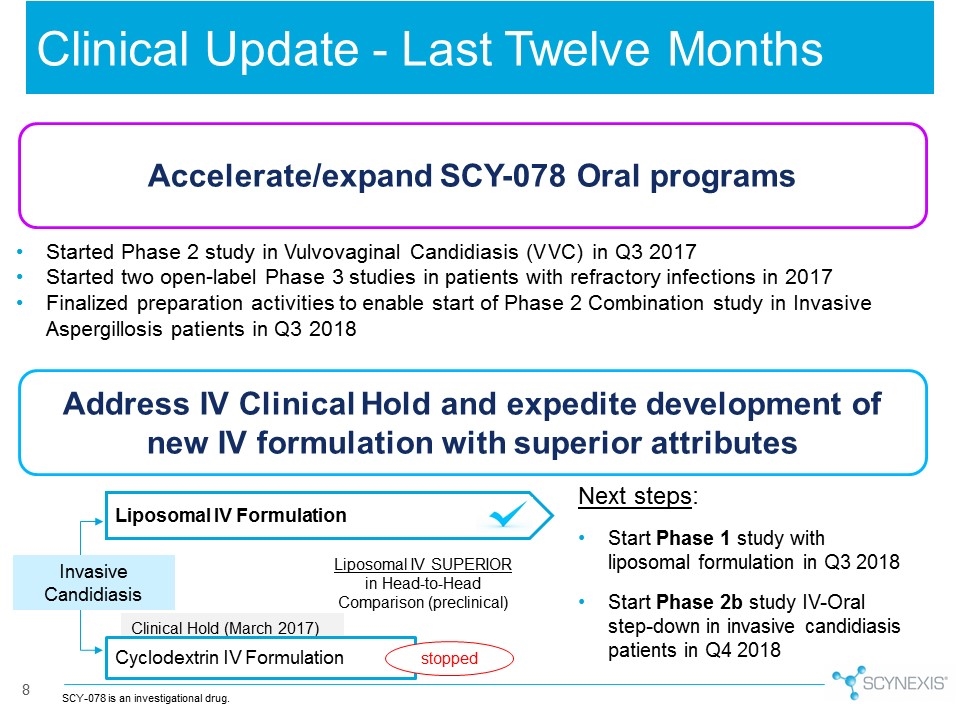
8 Clinical Update - Last Twelve Months Liposomal IV SUPERIOR in Head-to-Head Comparison (preclinical) Clinical Hold (March 2017) Cyclodextrin IV Formulation Liposomal IV Formulation Invasive Candidiasis stopped Accelerate/expand SCY-078 Oral programs Address IV Clinical Hold and expedite development of new IV formulation with superior attributes Next steps: Start Phase 1 study with liposomal formulation in Q3 2018 Start Phase 2b study IV-Oral step-down in invasive candidiasis patients in Q4 2018 Started Phase 2 study in Vulvovaginal Candidiasis (VVC) in Q3 2017 Started two open-label Phase 3 studies in patients with refractory infections in 2017 Finalized preparation activities to enable start of Phase 2 Combination study in Invasive Aspergillosis patients in Q3 2018 SCY-078 is an investigational drug.
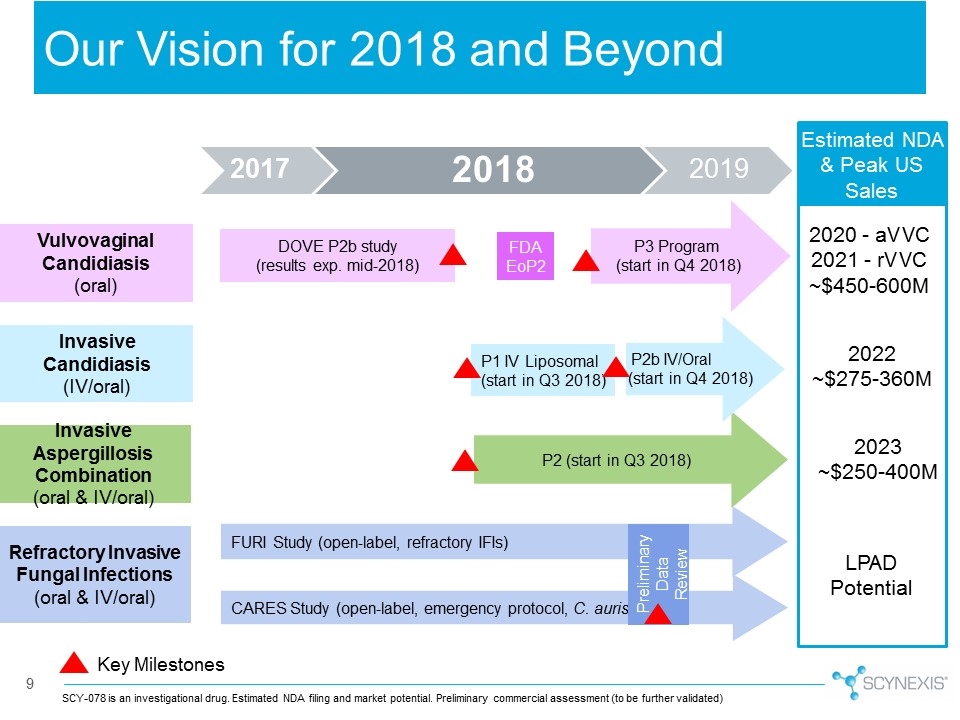
Our Vision for 2018 and Beyond 9 SCY-078 is an investigational drug. Vulvovaginal Candidiasis (oral) Invasive Aspergillosis Combination (oral & IV/oral) Refractory Invasive Fungal Infections (oral & IV/oral) CARES Study (open-label, emergency protocol, C. auris) P2 (start in Q3 2018) P2b IV/Oral (start in Q4 2018) DOVE P2b study (results exp. mid-2018) FURI Study (open-label, refractory IFIs) Invasive Candidiasis (IV/oral) P1 IV Liposomal (start in Q3 2018) FDA EoP2 Preliminary Data Review P3 Program (start in Q4 2018) Key Milestones Estimated NDA & Peak US Sales 2020 - aVVC 2021 - rVVC ~$450-600M 2022 ~$275-360M 2023 ~$250-400M LPAD Potential Estimated NDA filing and market potential. Preliminary commercial assessment (to be further validated) 2017 2019 2018

Vulvovaginal Candidiasis (VVC) SCY-078 – First of a Novel Oral/IV Triterpenoid Antifungal Family
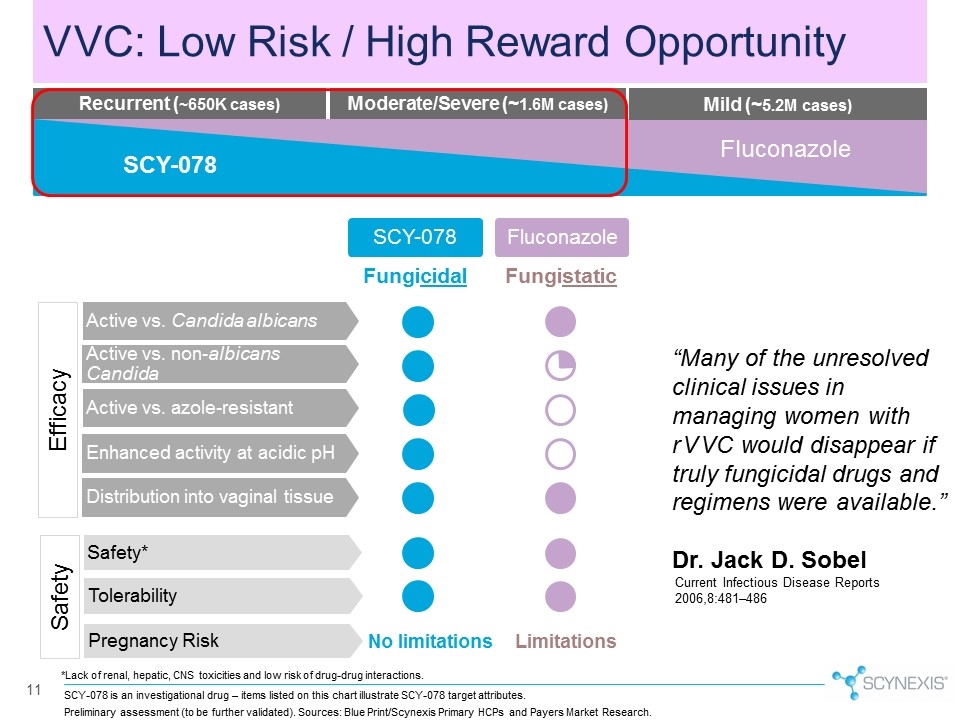
SCY-078 is an investigational drug – items listed on this chart illustrate SCY-078 target attributes. 11 VVC: Low Risk / High Reward Opportunity *Lack of renal, hepatic, CNS toxicities and low risk of drug-drug interactions. Pregnancy Risk SCY-078 Active vs. Candida albicans Active vs. non-albicans Candida Active vs. azole-resistant Tolerability Fluconazole Enhanced activity at acidic pH Distribution into vaginal tissue No limitations Limitations Fungicidal Fungistatic Safety* Efficacy Safety “Many of the unresolved clinical issues in managing women with rVVC would disappear if truly fungicidal drugs and regimens were available.” Dr. Jack D. Sobel Current Infectious Disease Reports 2006,8:481–486 Moderate/Severe (~1.6M cases) Mild (~5.2M cases) Recurrent (~650K cases) Fluconazole SCY-078 Preliminary assessment (to be further validated). Sources: Blue Print/Scynexis Primary HCPs and Payers Market Research.
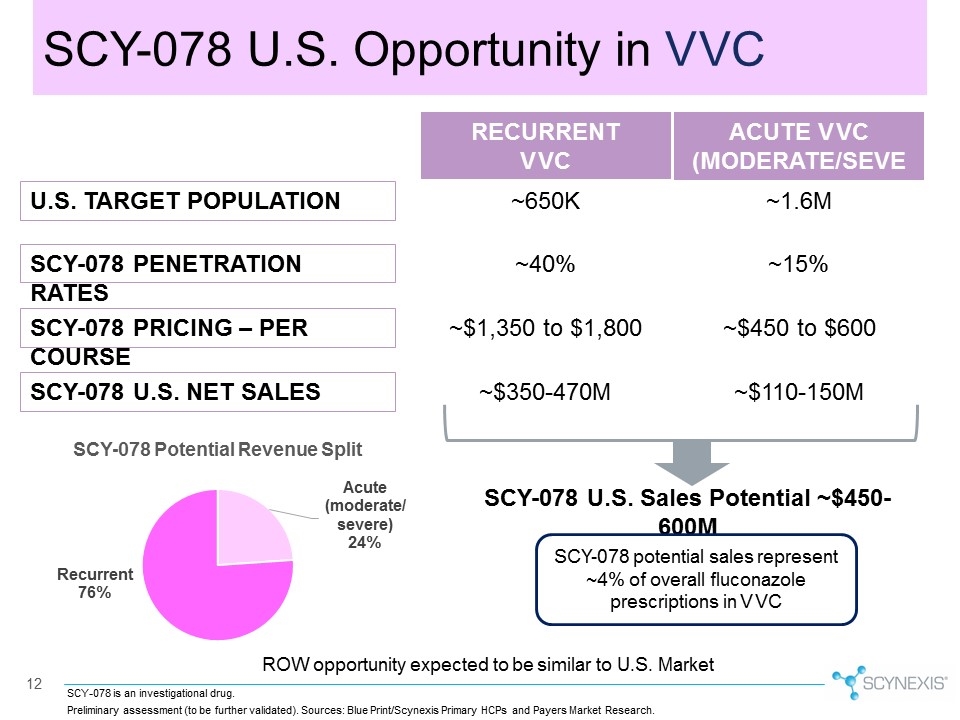
12 SCY-078 U.S. Opportunity in VVC SCY-078 U.S. Sales Potential ~$450-600M SCY-078 potential sales represent ~4% of overall fluconazole prescriptions in VVC ROW opportunity expected to be similar to U.S. Market U.S. TARGET POPULATION SCY-078 PENETRATION RATES SCY-078 PRICING – PER COURSE SCY-078 U.S. NET SALES ACUTE VVC (MODERATE/SEVERE) RECURRENT VVC ~1.6M ~650K ~15% ~40% ~$450 to $600 ~$1,350 to $1,800 ~$110-150M ~$350-470M SCY-078 is an investigational drug. Preliminary assessment (to be further validated). Sources: Blue Print/Scynexis Primary HCPs and Payers Market Research.
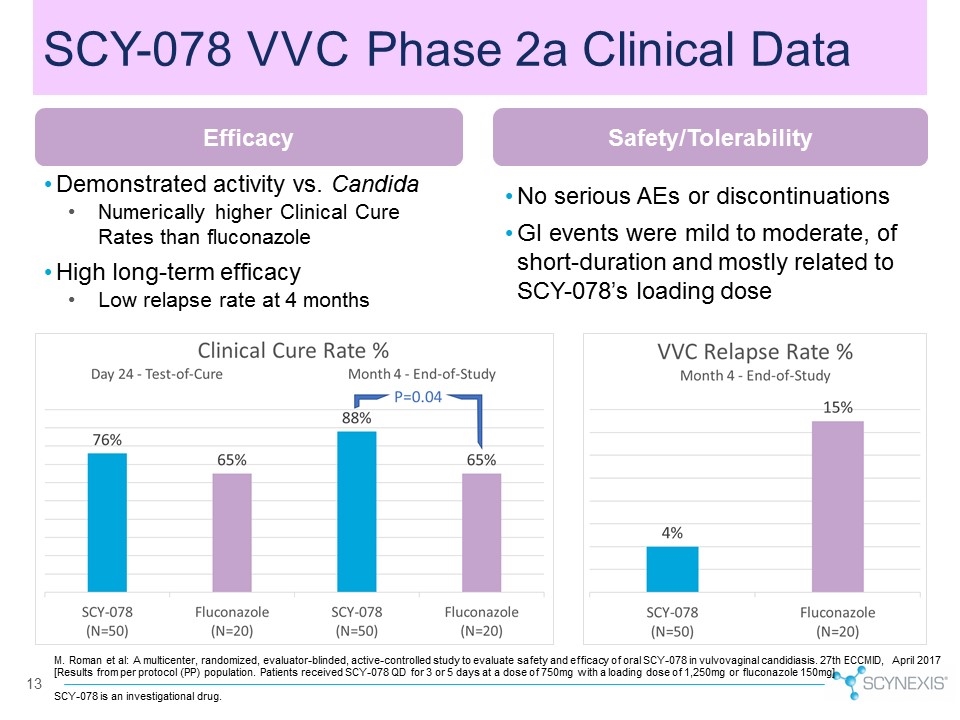
13 Demonstrated activity vs. Candida Numerically higher Clinical Cure Rates than fluconazole High long-term efficacy Low relapse rate at 4 months No serious AEs or discontinuations GI events were mild to moderate, of short-duration and mostly related to SCY-078’s loading dose M. Roman et al: A multicenter, randomized, evaluator-blinded, active-controlled study to evaluate safety and efficacy of oral SCY-078 in vulvovaginal candidiasis. 27th ECCMID, April 2017 [Results from per protocol (PP) population. Patients received SCY-078 QD for 3 or 5 days at a dose of 750mg with a loading dose of 1,250mg or fluconazole 150mg] SCY-078 VVC Phase 2a Clinical Data Efficacy Safety/Tolerability SCY-078 is an investigational drug.
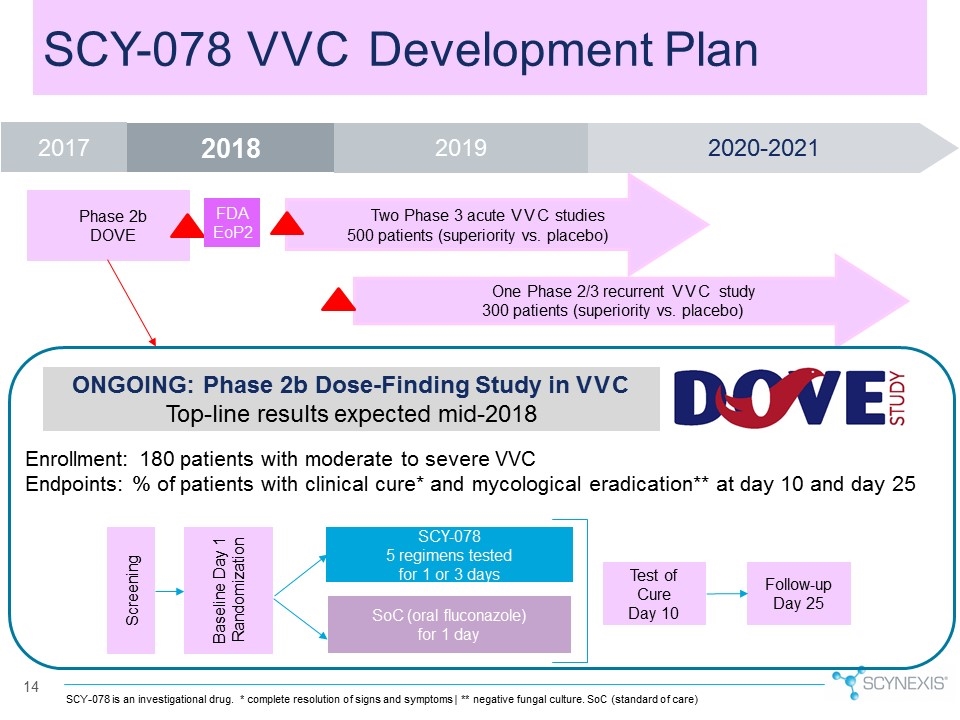
14 ONGOING: Phase 2b Dose-Finding Study in VVC Top-line results expected mid-2018 Enrollment: 180 patients with moderate to severe VVC Endpoints: % of patients with clinical cure* and mycological eradication** at day 10 and day 25 SCY-078 VVC Development Plan Baseline Day 1 Randomization Screening SCY-078 5 regimens tested for 1 or 3 days SoC (oral fluconazole) for 1 day Test of Cure Day 10 Follow-up Day 25 SCY-078 is an investigational drug. * complete resolution of signs and symptoms | ** negative fungal culture. SoC (standard of care) Two Phase 3 acute VVC studies 500 patients (superiority vs. placebo) One Phase 2/3 recurrent VVC study 300 patients (superiority vs. placebo) Phase 2b DOVE 2019 2018 2020-2021 FDA EoP2 2017

Invasive Candidiasis (IC) SCY-078 – First of a Novel Oral/IV Triterpenoid Antifungal Family
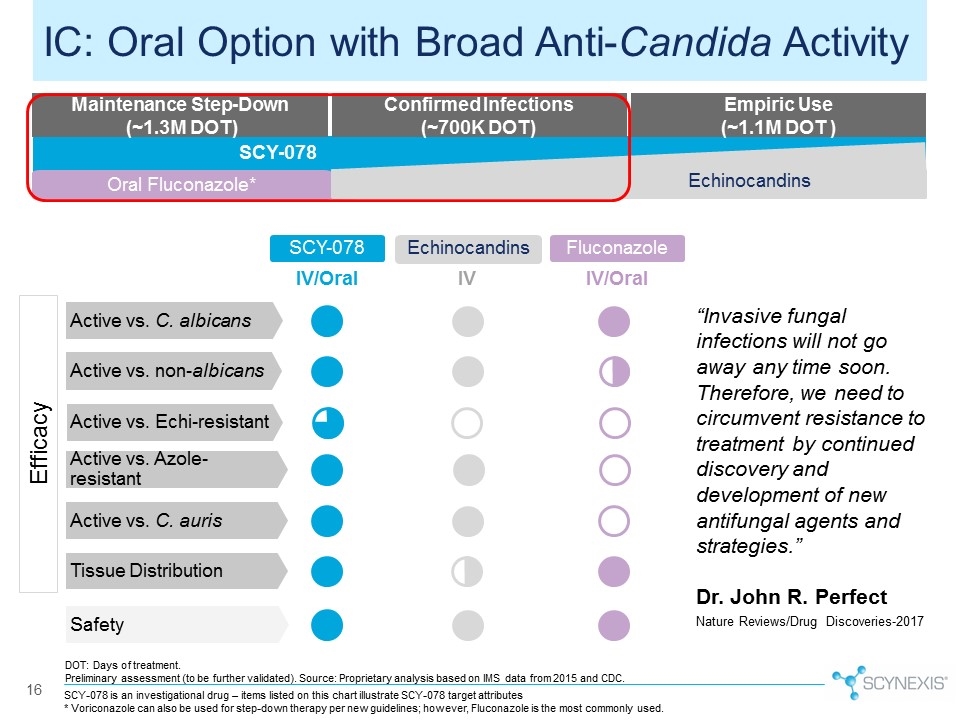
SCY-078 is an investigational drug – items listed on this chart illustrate SCY-078 target attributes * Voriconazole can also be used for step-down therapy per new guidelines; however, Fluconazole is the most commonly used. 16 IC: Oral Option with Broad Anti-Candida Activity Safety SCY-078 Active vs. C. albicans Active vs. non-albicans Active vs. Azole-resistant Echinocandins Tissue Distribution IV/Oral IV Fluconazole IV/Oral Active vs. Echi-resistant Active vs. C. auris Efficacy “Invasive fungal infections will not go away any time soon. Therefore, we need to circumvent resistance to treatment by continued discovery and development of new antifungal agents and strategies.” Dr. John R. Perfect Nature Reviews/Drug Discoveries-2017 Maintenance Step-Down (~1.3M DOT) Confirmed Infections (~700K DOT) Empiric Use (~1.1M DOT ) Oral Fluconazole* SCY-078 Echinocandins Echinocandins DOT: Days of treatment. Preliminary assessment (to be further validated). Source: Proprietary analysis based on IMS data from 2015 and CDC.
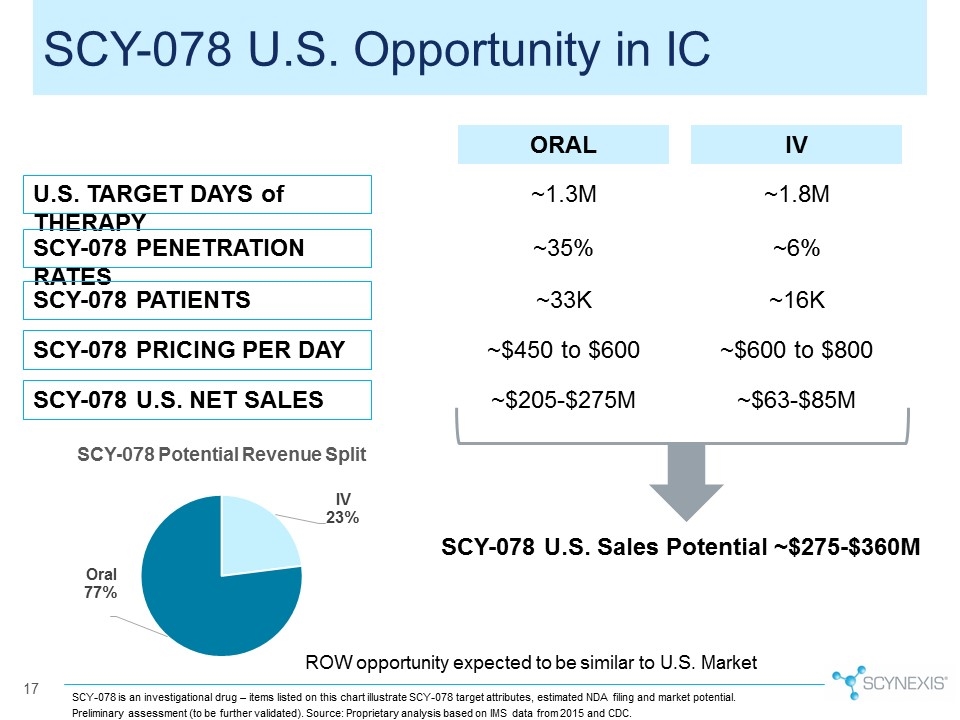
17 SCY-078 U.S. Opportunity in IC U.S. TARGET DAYS of THERAPY SCY-078 PENETRATION RATES SCY-078 PRICING PER DAY SCY-078 U.S. NET SALES IV ORAL ~1.8M ~1.3M ~6% ~35% ~$600 to $800 ~$450 to $600 ~$63-$85M ~$205-$275M SCY-078 U.S. Sales Potential ~$275-$360M ROW opportunity expected to be similar to U.S. Market SCY-078 is an investigational drug – items listed on this chart illustrate SCY-078 target attributes, estimated NDA filing and market potential. SCY-078 PATIENTS ~16K ~33K Preliminary assessment (to be further validated). Source: Proprietary analysis based on IMS data from 2015 and CDC.
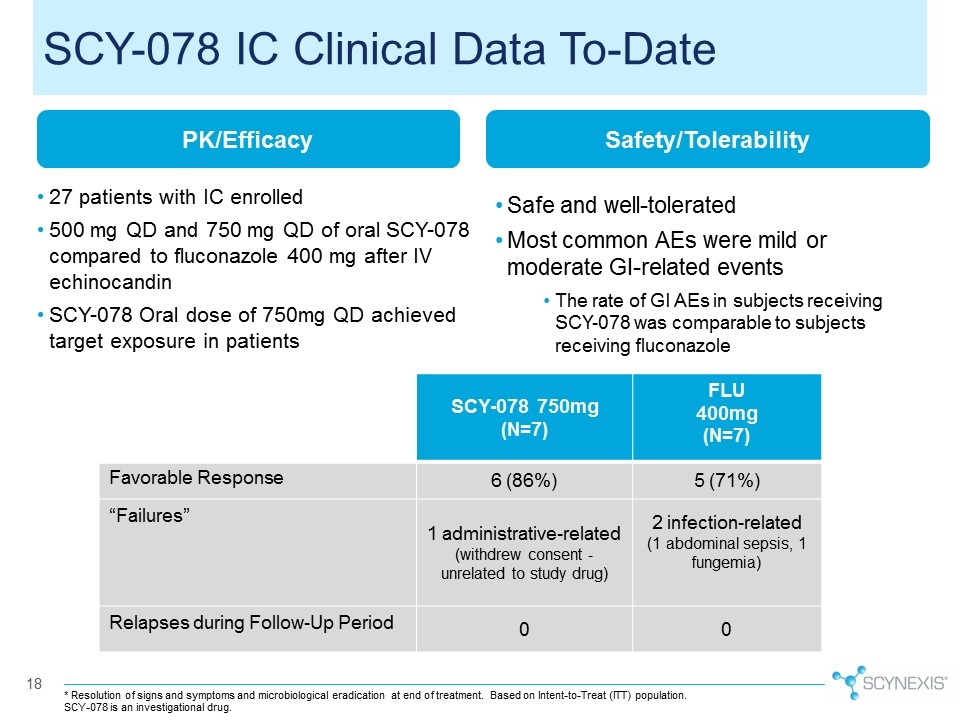
18 PK/Efficacy 27 patients with IC enrolled 500 mg QD and 750 mg QD of oral SCY-078 compared to fluconazole 400 mg after IV echinocandin SCY-078 Oral dose of 750mg QD achieved target exposure in patients Safety/Tolerability Safe and well-tolerated Most common AEs were mild or moderate GI-related events The rate of GI AEs in subjects receiving SCY-078 was comparable to subjects receiving fluconazole SCY-078 750mg (N=7) FLU 400mg (N=7) Favorable Response 6 (86%) 5 (71%) “Failures” 1 administrative-related (withdrew consent - unrelated to study drug) 2 infection-related (1 abdominal sepsis, 1 fungemia) Relapses during Follow-Up Period 0 0 * Resolution of signs and symptoms and microbiological eradication at end of treatment. Based on Intent-to-Treat (ITT) population. SCY-078 IC Clinical Data To-Date SCY-078 is an investigational drug.
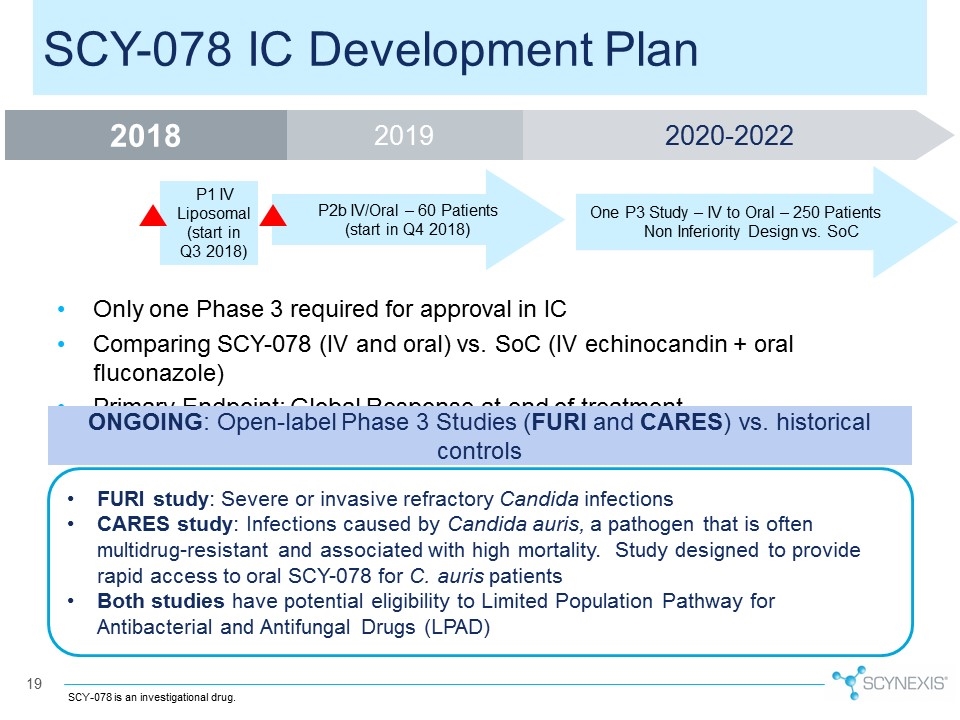
Only one Phase 3 required for approval in IC Comparing SCY-078 (IV and oral) vs. SoC (IV echinocandin + oral fluconazole) Primary Endpoint: Global Response at end of treatment 19 SCY-078 IC Development Plan ONGOING: Open-label Phase 3 Studies (FURI and CARES) vs. historical controls FURI study: Severe or invasive refractory Candida infections CARES study: Infections caused by Candida auris, a pathogen that is often multidrug-resistant and associated with high mortality. Study designed to provide rapid access to oral SCY-078 for C. auris patients Both studies have potential eligibility to Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) SCY-078 is an investigational drug. P2b IV/Oral – 60 Patients (start in Q4 2018) P1 IV Liposomal (start in Q3 2018) One P3 Study – IV to Oral – 250 Patients Non Inferiority Design vs. SoC 2019 2018 2020-2022

Invasive Aspergillosis (IA) SCY-078 – First of a Novel Oral/IV Triterpenoid Antifungal Family
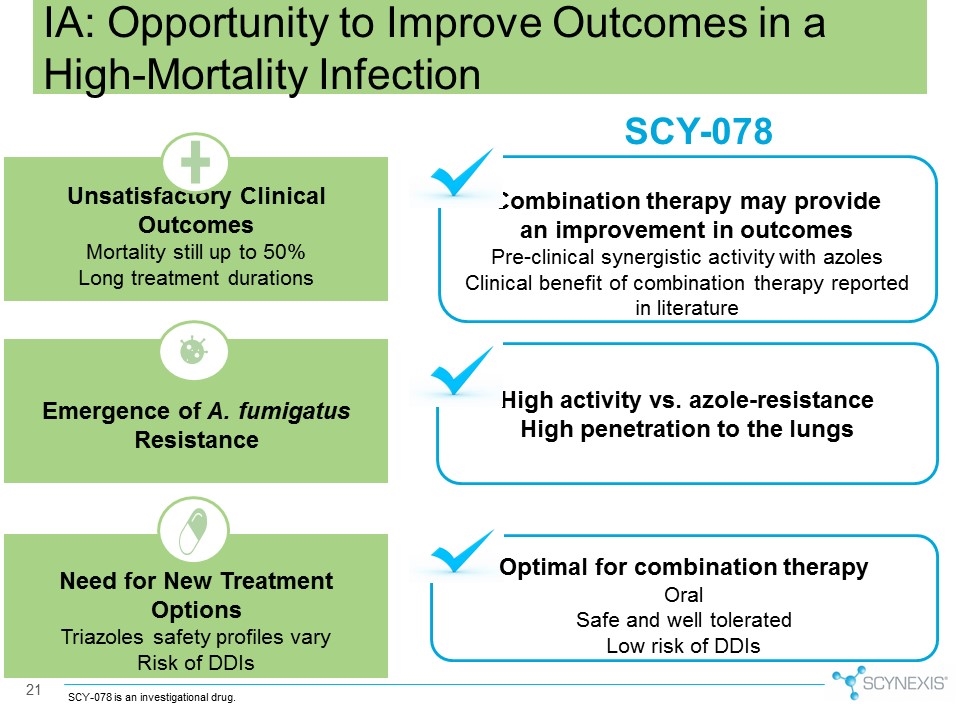
21 SCY-078 High activity vs. azole-resistance High penetration to the lungs Optimal for combination therapy Oral Safe and well tolerated Low risk of DDIs Emergence of A. fumigatus Resistance Need for New Treatment Options Triazoles safety profiles vary Risk of DDIs Unsatisfactory Clinical Outcomes Mortality still up to 50% Long treatment durations IA: Opportunity to Improve Outcomes in a High-Mortality Infection SCY-078 is an investigational drug. Combination therapy may provide an improvement in outcomes Pre-clinical synergistic activity with azoles Clinical benefit of combination therapy reported in literature
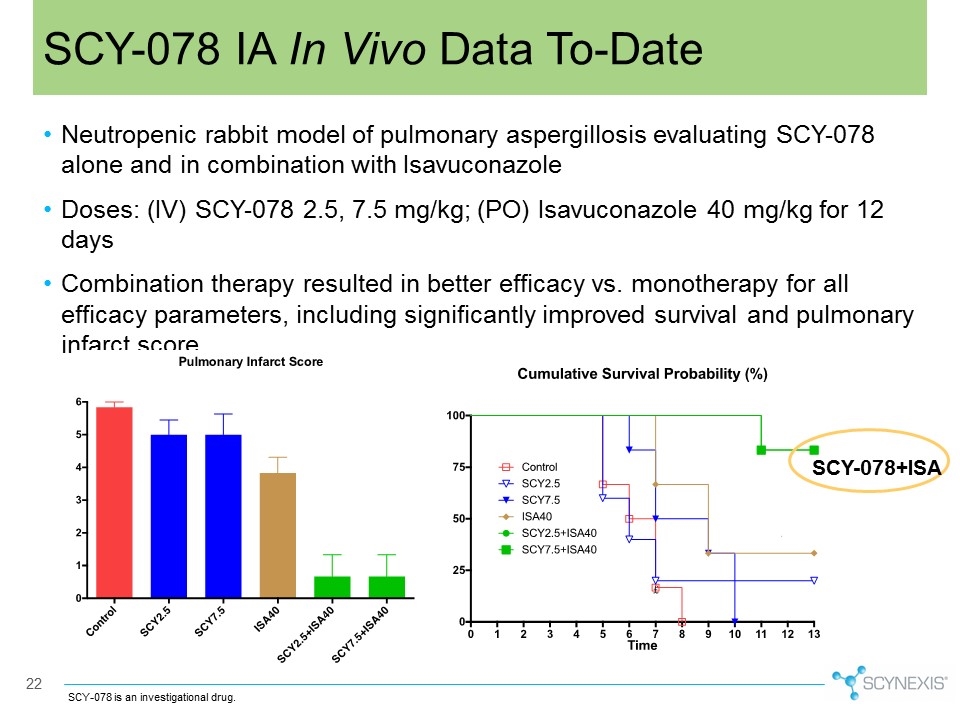
Neutropenic rabbit model of pulmonary aspergillosis evaluating SCY-078 alone and in combination with Isavuconazole Doses: (IV) SCY-078 2.5, 7.5 mg/kg; (PO) Isavuconazole 40 mg/kg for 12 days Combination therapy resulted in better efficacy vs. monotherapy for all efficacy parameters, including significantly improved survival and pulmonary infarct score 22 SCY-078 IA In Vivo Data To-Date SCY-078 is an investigational drug. SCY-078+ISA
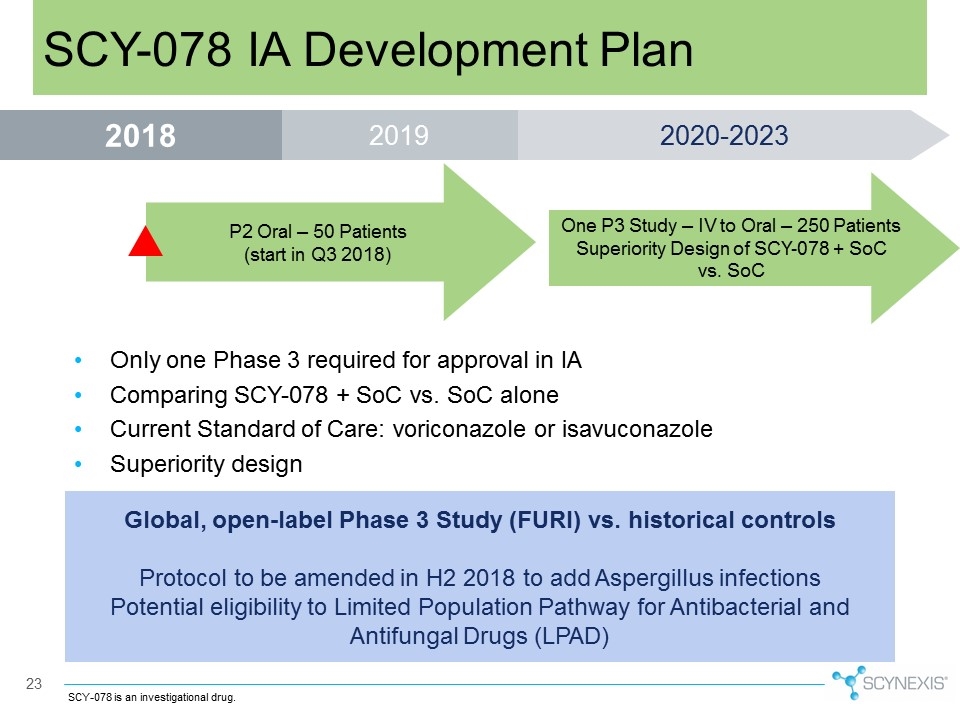
23 SCY-078 IA Development Plan Global, open-label Phase 3 Study (FURI) vs. historical controls Protocol to be amended in H2 2018 to add Aspergillus infections Potential eligibility to Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) SCY-078 is an investigational drug. Only one Phase 3 required for approval in IA Comparing SCY-078 + SoC vs. SoC alone Current Standard of Care: voriconazole or isavuconazole Superiority design P2 Oral – 50 Patients (start in Q3 2018) One P3 Study – IV to Oral – 250 Patients Superiority Design of SCY-078 + SoC vs. SoC 2019 2018 2020-2023

Conclusion SCY-078 – First of a Novel Oral/IV Triterpenoid Antifungal Family

SCYX: Key Milestones Recent Milestones Initiated Phase 2b DOVE study in VVC(Q3 2017) Accelerated development of new IV liposomal formulation Two on-going clinical trials for refractory infections (FURI & CARES) Upcoming Milestones Mid-2018: Phase 2b DOVE study read-out Q3 2018: Initiate Phase 2 Combo study in IA Q3 2018: Initiate Phase 1 study with new liposomal IV Q4 2018: Initiate VVC Phase 3 program Q4 2018: Initiate Phase 2 study in IC Q4 2018: Conduct FURI & CARES Preliminary Data Review Indicate key target milestones for 2018.

Positive track record in drug development & antifungal expertise CEO: Marco Taglietti, M.D. Schering-Plough, Stiefel, Forest Labs CMO: David Angulo, M.D. Schering-Plough, Stiefel, Brickell Biotech CFO: Eric Francois Cowen, Lazard, Topi General Counsel: Scott Sukenick Cooley Diverse backgrounds & operating experience in healthcare Guy Macdonald, Chairman (Tetraphase, Merck) Steven Gilman, Ph.D. (Contrafect, Cubist) Ann Hanham, Ph.D. (BAR Capital, Burrill, FDA) David Hastings (Unilife, Incyte) Patrick Machado (Medivation) Marion McCourt (Regeneron, Medivation, Amgen) SCYX: Experienced Management LEADERSHIP BOARD OF DIRECTORS
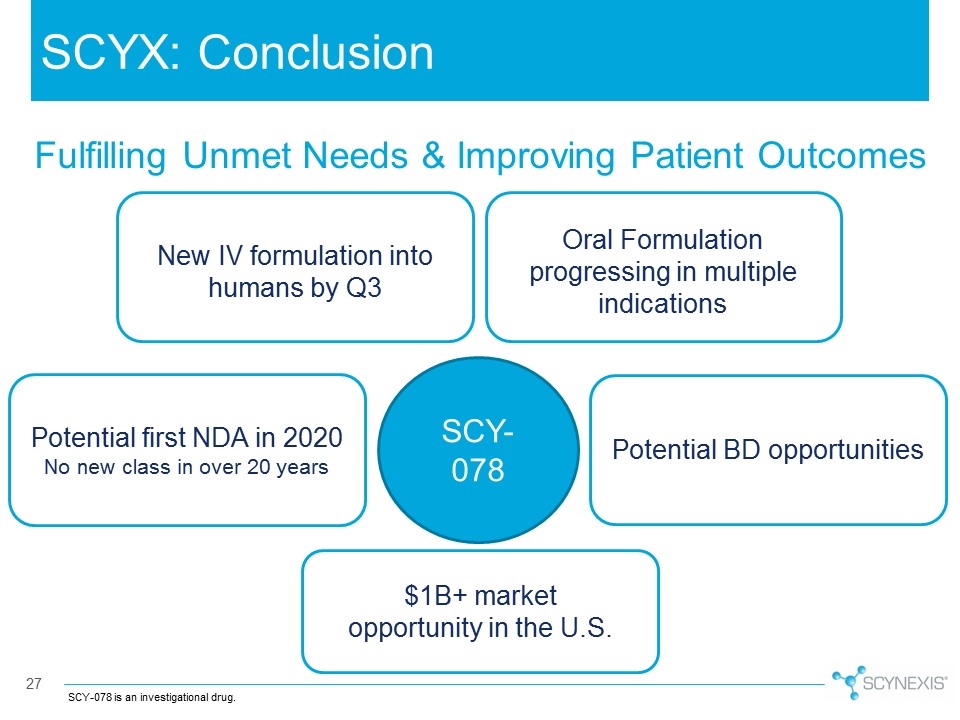
SCYX: Conclusion SCY-078 is an investigational drug. $1B+ market opportunity in the U.S. Potential BD opportunities SCY-078 Potential first NDA in 2020 No new class in over 20 years New IV formulation into humans by Q3 Fulfilling Unmet Needs & Improving Patient Outcomes Oral Formulation progressing in multiple indications

Thank You SCY-078 – First of a Novel Oral/IV Triterpenoid Antifungal Family