

SCY-078 – First Representative of a Novel Oral/IV Triterpenoid Antifungal Family CORPORATE PRESENTATION | January 2018
Certain statements regarding SCYNEXIS, Inc. (the “Company”) made in this presentation constitute forward-looking statements, including, but not limited to, statements regarding our business strategies and goals, plans and prospects, market size, adoption rate, potential revenue, clinical validity and utility, growth opportunities, future products and product pipeline. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from our expectations. These risks and uncertainties include, but are not limited, to: risks inherent in SCYNEXIS' ability to successfully develop SCY-078, including SCYNEXIS' ability to resolve the FDA's concerns regarding the IV formulation of SCY-078 on a timely basis, if at all, and obtain FDA’s approval for SCY-078; the expected costs of studies and when they might begin or be concluded; and SCYNEXIS' reliance on third parties to conduct SCYNEXIS' clinical studies. Forward-looking statements may be identified by the use of the words “anticipates,” “expects,” “intends,” “plans,” “could,” “should,” “would,” “may,” “will,” “believes,” “estimates,” “potential,” or “continue” and variations or similar expressions. These statements are based upon the current expectations and beliefs of management and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include, but are not limited to, risks and uncertainties discussed in the Company's most recent reports filed with the Securities and Exchange Commission ("SEC") including under the caption “Risks Factors” in the Company’s annual report on Form 10-K, which factors are incorporated herein by reference. Readers are cautioned not to place undue reliance on any of these forward-looking statements. The Company undertakes no obligation to update any of these forward-looking statements to reflect events or circumstances after the date of this presentation, or to reflect actual outcomes. Forward-Looking Statements
SCYX: Investment Opportunity A novel triterpenoid oral & IV broad spectrum antifungal EXPERIENCED TEAM SCY-078 FAVORABLE TECHNICALS Committed to positively impacting the lives of patients suffering from difficult-to-treat and often life-threatening infections

Positive track record in drug development & antifungal expertise CEO: Marco Taglietti, M.D. Schering-Plough, Stiefel, Forest Labs CMO: David Angulo, M.D. Schering-Plough, Stiefel, Brickell Biotech CFO: Eric Francois Cowen, Lazard, Topi General Counsel: Scott Sukenick Cooley Diverse backgrounds & operating experience Guy Macdonald, Chairman (Tetraphase, Merck) Steven Gilman, Ph.D. (Contrafect, Cubist) Ann Hanham, Ph.D. (BAR Capital, Burrill) David Hastings (Unilife, Incyte) Patrick Machado (Medivation) Marion McCourt (Axovant, Medivation, Amgen) Foundation: Experienced Management LEADERSHIP BOARD OF DIRECTORS
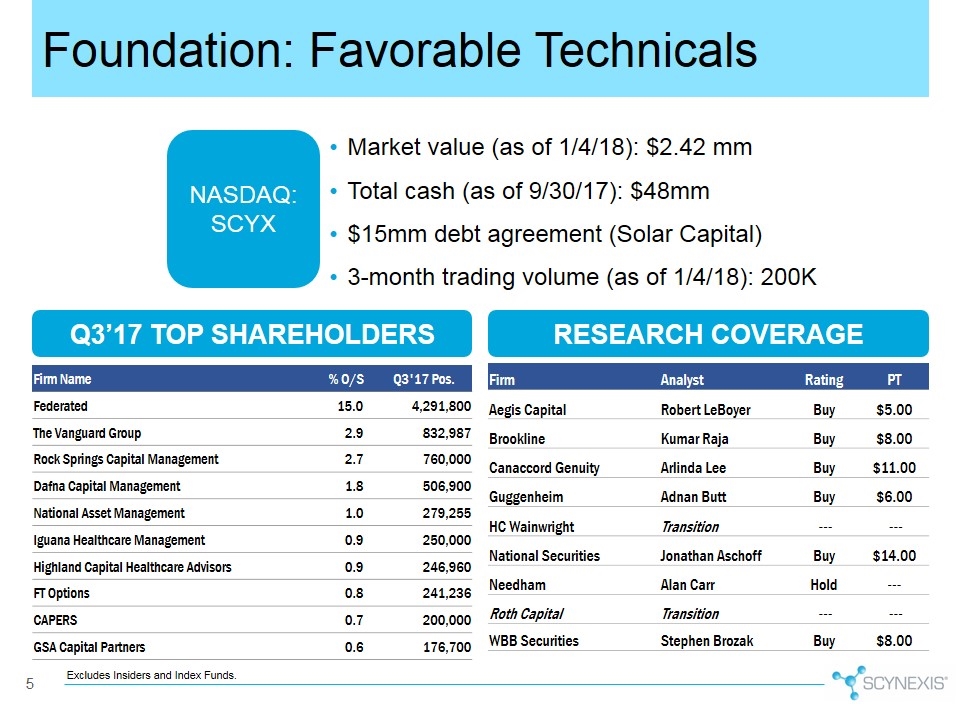
Market value (as of 1/4/18): $2.42 mm Total cash (as of 9/30/17): $48mm $15mm debt agreement (Solar Capital) 3-month trading volume (as of 1/4/18): 200K shares Foundation: Favorable Technicals Q3’17 TOP SHAREHOLDERS RESEARCH COVERAGE Excludes Insiders and Index Funds. NASDAQ: SCYX Firm Name % O/S Q3'17 Pos. Federated 15.0 4,291,800 The Vanguard Group 2.9 832,987 Rock Springs Capital Management 2.7 760,000 Dafna Capital Management 1.8 506,900 National Asset Management 1.0 279,255 Iguana Healthcare Management 0.9 250,000 Highland Capital Healthcare Advisors 0.9 246,960 FT Options 0.8 241,236 CAPERS 0.7 200,000 GSA Capital Partners 0.6 176,700 Firm Analyst Rating PT Aegis Capital Robert LeBoyer Buy $5.00 Brookline Kumar Raja Buy $8.00 Canaccord Genuity Arlinda Lee Buy $11.00 Guggenheim Adnan Butt Buy $6.00 HC Wainwright Transition --- --- National Securities Jonathan Aschoff Buy $14.00 Needham Alan Carr Hold --- Roth Capital Transition --- --- WBB Securities Stephen Brozak Buy $8.00
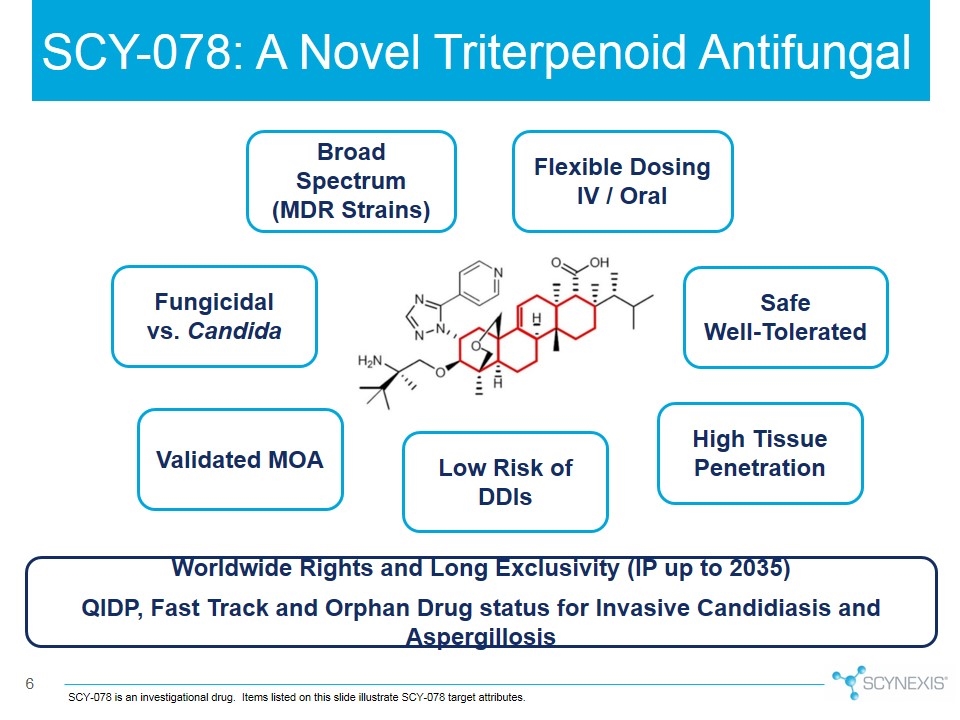
SCY-078: A Novel Triterpenoid Antifungal SCY-078 is an investigational drug. Items listed on this slide illustrate SCY-078 target attributes. Flexible Dosing IV / Oral Safe Well-Tolerated High Tissue Penetration Low Risk of DDIs Validated MOA Fungicidal vs. Candida Broad Spectrum (MDR Strains) Worldwide Rights and Long Exclusivity (IP up to 2035) QIDP, Fast Track and Orphan Drug status for Invasive Candidiasis and Aspergillosis
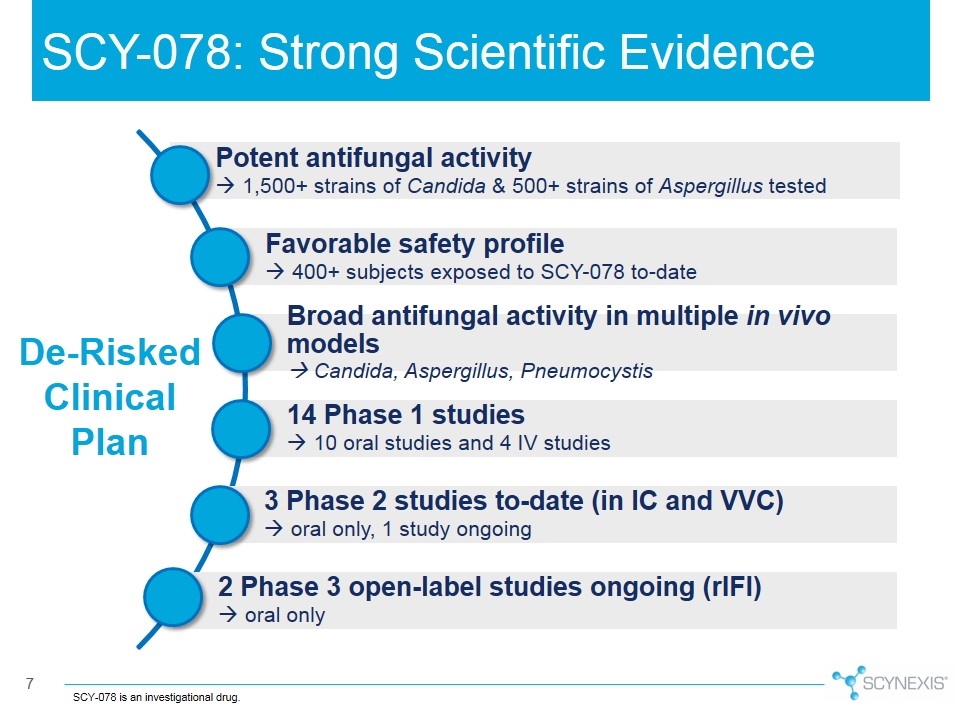
SCY-078: Strong Scientific Evidence SCY-078 is an investigational drug. De-Risked Clinical Plan 2 Phase 3 open-label studies ongoing ( rIFI ) à oral only Potent antifungal activity à 1,500+ strains of Candida & 500+ strains of Aspergillus tested Favorable safety profile à 400+ subjects exposed to SCY-078 to-date 3 Phase 2 studies to-date (in IC and VVC) à oral only, 1 study ongoing 14 Phase 1 studies à 10 oral studies and 4 IV studies Broad antifungal activity in multiple in vivo models à Candida, Aspergillus, Pneumocystis
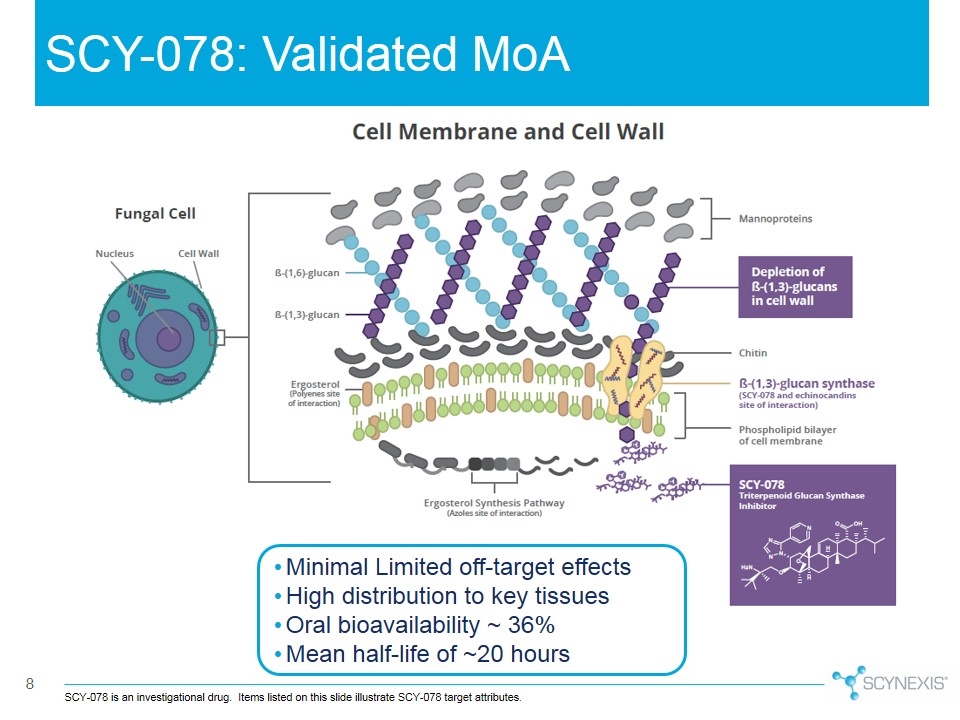
SCY-078: Validated MoA SCY-078 is an investigational drug. Items listed on this slide illustrate SCY-078 target attributes. Minimal Limited off-target effects High distribution to key tissues Oral bioavailability ~ 36% Mean half-life of ~20 hours
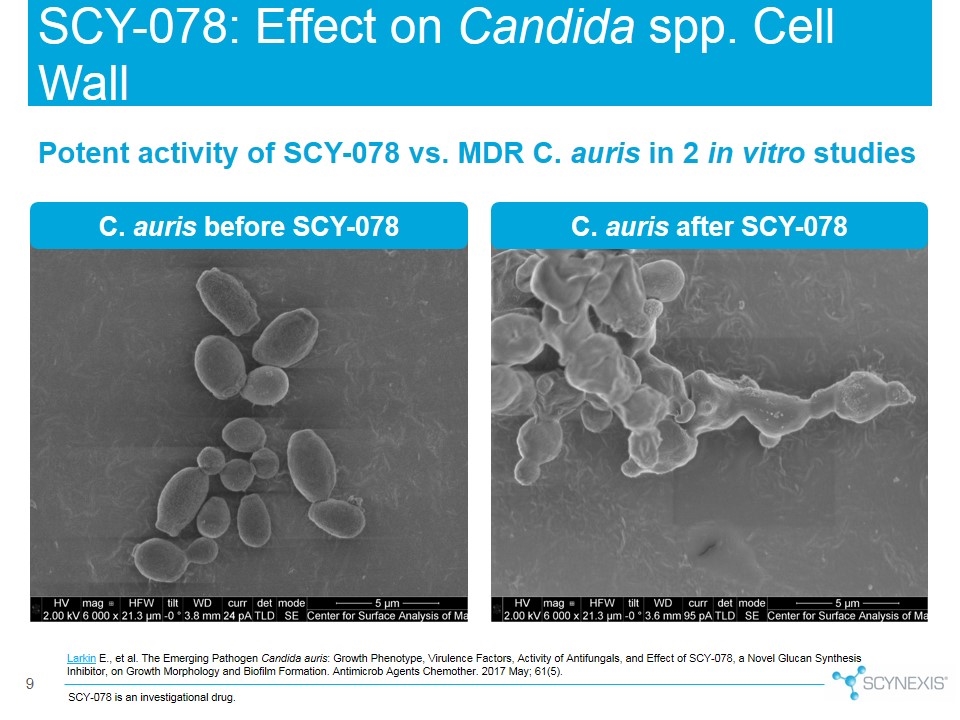
9 Larkin E., et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob Agents Chemother. 2017 May; 61(5). Potent activity of SCY-078 vs. MDR C. auris in 2 in vitro studies SCY-078: Effect on Candida spp. Cell Wall C. auris before SCY-078 C. auris after SCY-078 SCY-078 is an investigational drug.
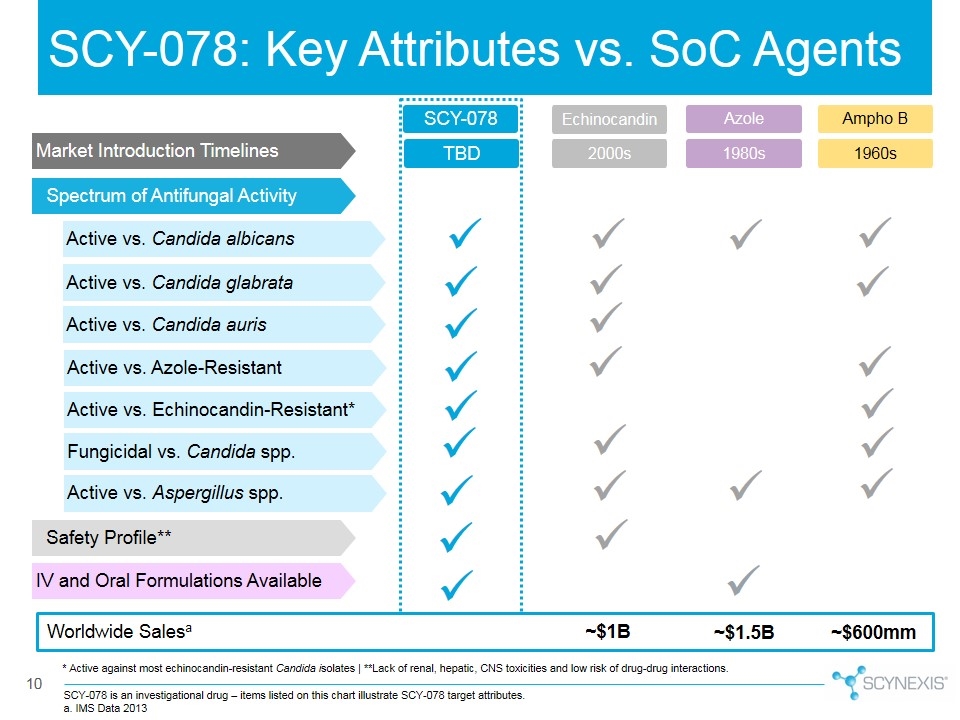
SCY-078 is an investigational drug – items listed on this chart illustrate SCY-078 target attributes. a. IMS Data 2013 Fungicidal vs. Candida spp. IV and Oral Formulations Available Azole SCY-078 Active vs. Candida albicans Active vs. Candida glabrata Active vs. Azole-Resistant Active vs. Echinocandin-Resistant* 10 Ampho B Echinocandin Spectrum of Antifungal Activity Safety Profile** ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü ü Active vs. Aspergillus spp. ü ü ü ü Worldwide Salesa ~$1B ~$1.5B ~$600mm SCY-078: Key Attributes vs. SoC Agents Market Introduction Timelines TBD 2000s 1980s 1960s Active vs. Candida auris * Active against most echinocandin-resistant Candida isolates | **Lack of renal, hepatic, CNS toxicities and low risk of drug-drug interactions. ü ü
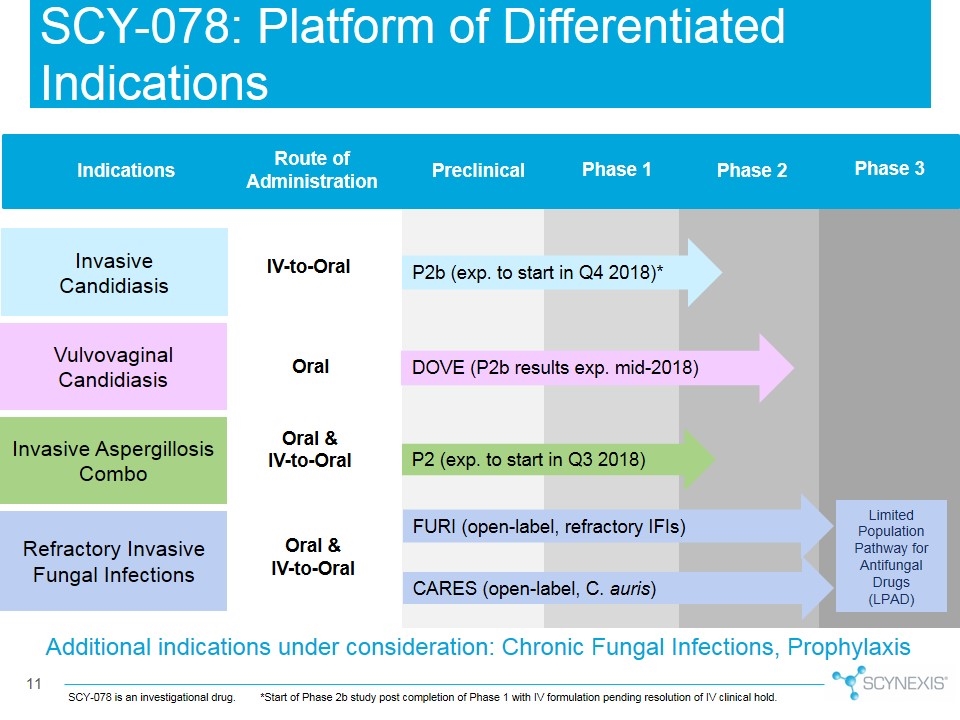
Additional indications under consideration: Chronic Fungal Infections, Prophylaxis Vulvovaginal Candidiasis Invasive Aspergillosis Combo Refractory Invasive Fungal Infections Preclinical Phase 1 Phase 2 Phase 3 CARES (open-label, C. auris) P2 (exp. to start in Q3 2018) P2b (exp. to start in Q4 2018)* DOVE (P2b results exp. mid-2018) Route of Administration Indications IV-to-Oral Oral & IV-to-Oral Oral Oral & IV-to-Oral FURI (open-label, refractory IFIs) SCY-078: Platform of Differentiated Indications Invasive Candidiasis Limited Population Pathway for Antifungal Drugs (LPAD) SCY-078 is an investigational drug.*Start of Phase 2b study post completion of Phase 1 with IV formulation pending resolution of IV clinical hold.
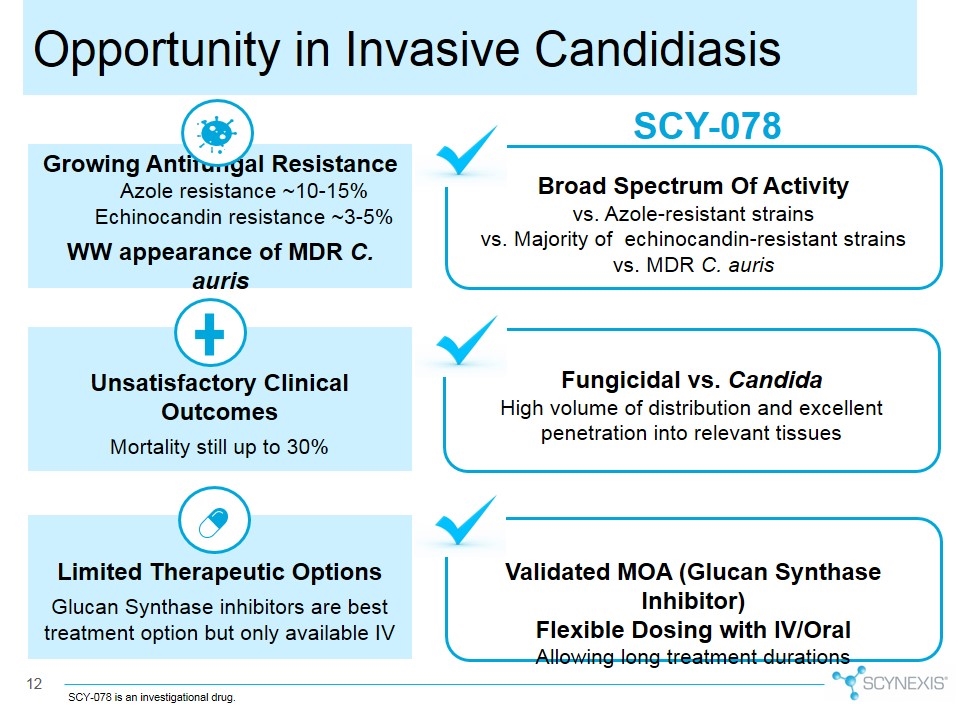
SCY-078 Broad Spectrum Of Activity vs. Azole-resistant strains vs. Majority of echinocandin-resistant strains vs. MDR C. auris Fungicidal vs. Candida High volume of distribution and excellent penetration into relevant tissues Validated MOA (Glucan Synthase Inhibitor) Flexible Dosing with IV/Oral Allowing long treatment durations Growing Antifungal Resistance Azole resistance ~10-15% Echinocandin resistance ~3-5% WW appearance of MDR C. auris Limited Therapeutic Options Glucan Synthase inhibitors are best treatment option but only available IV Unsatisfactory Clinical Outcomes Mortality still up to 30% Opportunity in Invasive Candidiasis SCY-078 is an investigational drug.
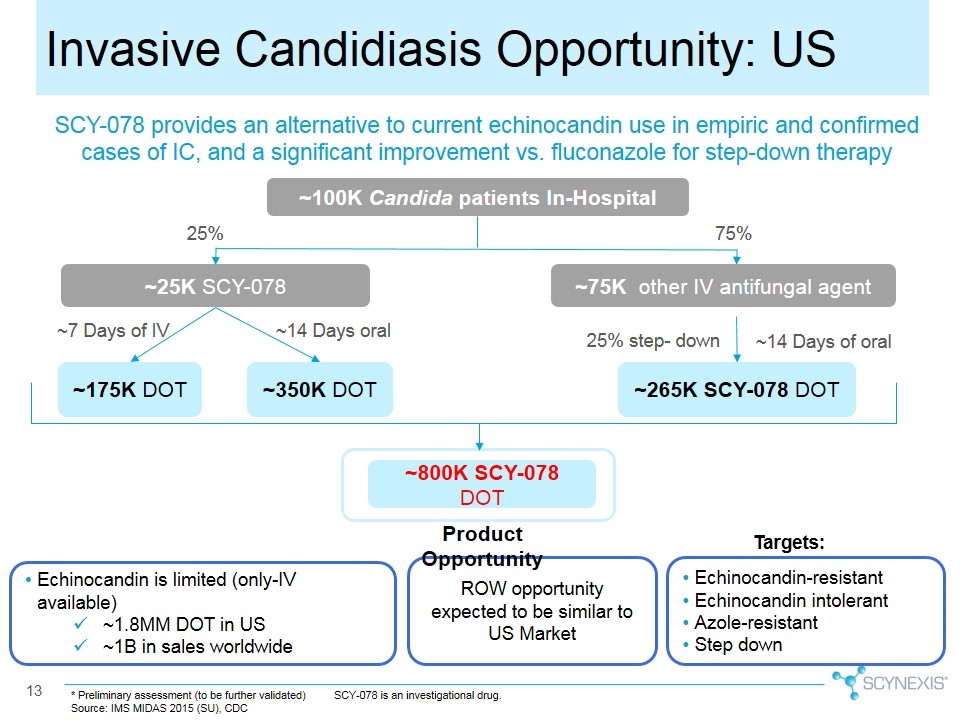
Invasive Candidiasis Opportunity: US * Preliminary assessment (to be further validated) Source: IMS MIDAS 2015 (SU), CDC SCY-078 is an investigational drug. SCY-078 provides an alternative to current echinocandin use in empiric and confirmed cases of IC, and a significant improvement vs. fluconazole for step-down therapy ROW opportunity expected to be similar to US Market ~100K Candida patients In-Hospital ~25K SCY-078 ~175K DOT ~800K SCY-078 DOT 25% ~75K other IV antifungal agent 75% ~7 Days of IV ~350K DOT ~265K SCY-078 DOT ~14 Days of oral 25% step- down ~14 Days oral Product Opportunity Echinocandin-resistant Echinocandin intolerant Azole-resistant Step down Echinocandin is limited (only-IV available) ~1.8MM DOT in US ~1B in sales worldwide Targets:
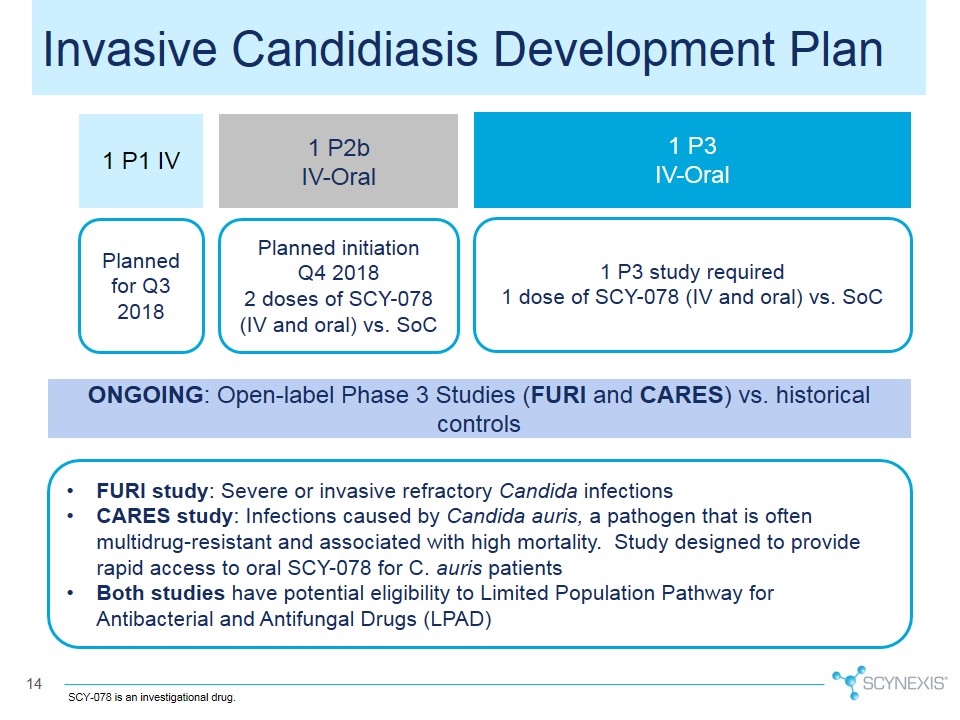
Invasive Candidiasis Development Plan 1 P2b IV-Oral 1 P1 IV 1 P3 IV-Oral Planned for Q3 2018 Planned initiation Q4 2018 2 doses of SCY-078 (IV and oral) vs. SoC 1 P3 study required 1 dose of SCY-078 (IV and oral) vs. SoC ONGOING: Open-label Phase 3 Studies (FURI and CARES) vs. historical controls FURI study: Severe or invasive refractory Candida infections CARES study: Infections caused by Candida auris, a pathogen that is often multidrug-resistant and associated with high mortality. Study designed to provide rapid access to oral SCY-078 for C. auris patients Both studies have potential eligibility to Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) SCY-078 is an investigational drug.
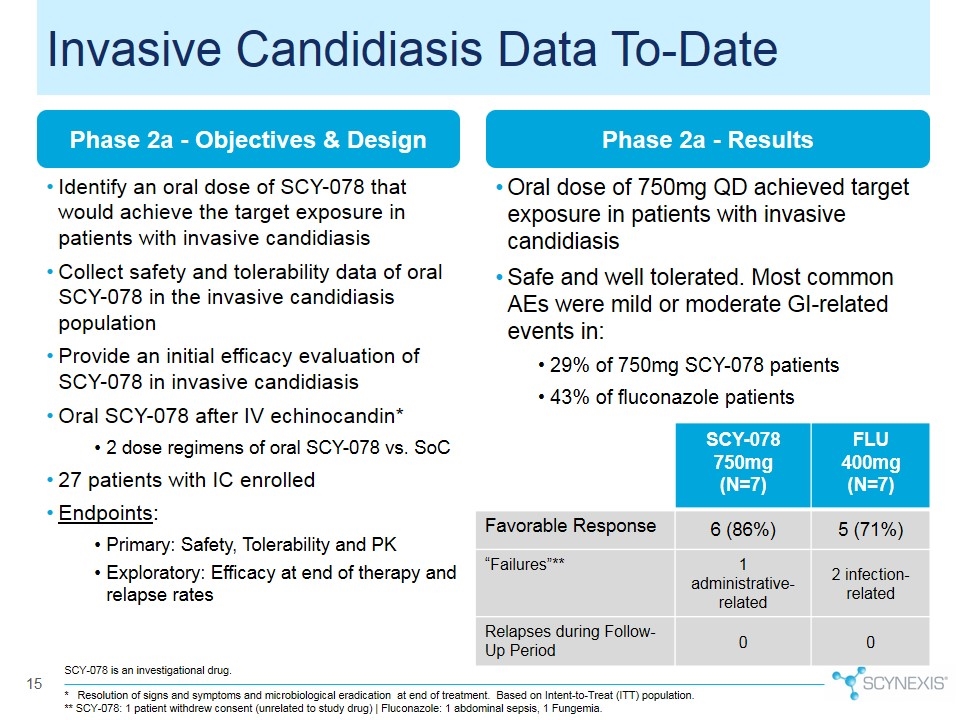
Phase 2a - Objectives & Design Identify an oral dose of SCY-078 that would achieve the target exposure in patients with invasive candidiasis Collect safety and tolerability data of oral SCY-078 in the invasive candidiasis population Provide an initial efficacy evaluation of SCY-078 in invasive candidiasis Oral SCY-078 after IV echinocandin* 2 dose regimens of oral SCY-078 vs. SoC 27 patients with IC enrolled Endpoints: Primary: Safety, Tolerability and PK Exploratory: Efficacy at end of therapy and relapse rates Phase 2a - Results Oral dose of 750mg QD achieved target exposure in patients with invasive candidiasis Safe and well tolerated. Most common AEs were mild or moderate GI-related events in: 29% of 750mg SCY-078 patients 43% of fluconazole patients SCY-078 750mg (N=7) FLU 400mg (N=7) Favorable Response 6 (86%) 5 (71%) “Failures”** 1 administrative-related 2 infection-related Relapses during Follow-Up Period 0 0 * Resolution of signs and symptoms and microbiological eradication at end of treatment. Based on Intent-to-Treat (ITT) population. ** SCY-078: 1 patient withdrew consent (unrelated to study drug) | Fluconazole: 1 abdominal sepsis, 1 Fungemia. Invasive Candidiasis Data To-Date SCY-078 is an investigational drug.
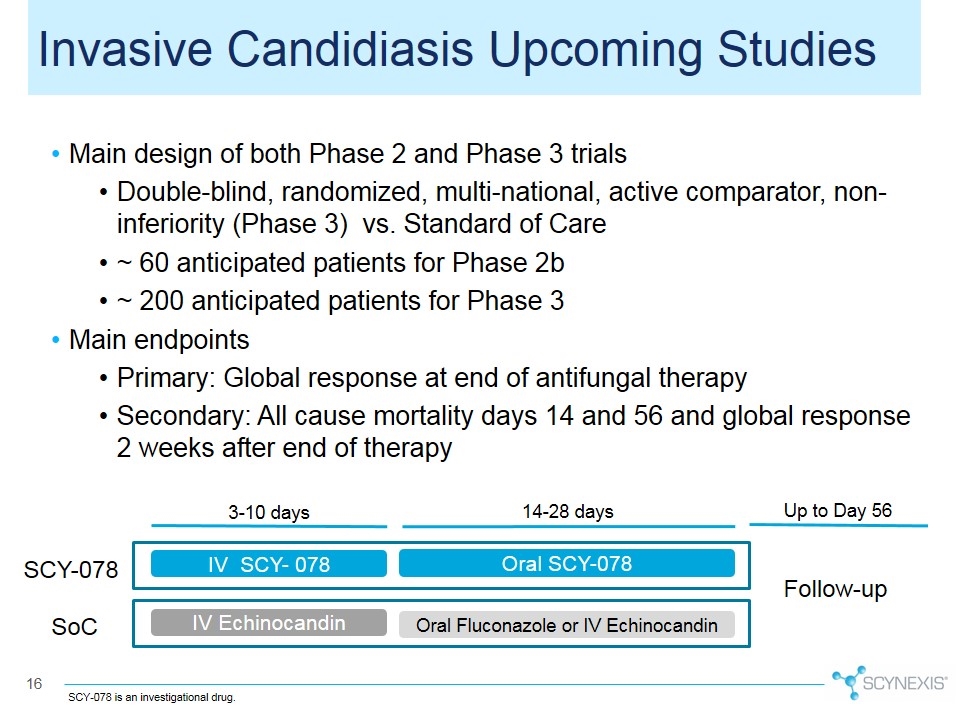
Main design of both Phase 2 and Phase 3 trials Double-blind, randomized, multi-national, active comparator, non-inferiority (Phase 3) vs. Standard of Care ~ 60 anticipated patients for Phase 2b ~ 200 anticipated patients for Phase 3 Main endpoints Primary: Global response at end of antifungal therapy Secondary: All cause mortality days 14 and 56 and global response 2 weeks after end of therapy IV SCY- 078 Oral SCY-078 IV Echinocandin Oral Fluconazole or IV Echinocandin Follow-up 3-10 days 14-28 days Up to Day 56 Invasive Candidiasis Upcoming Studies SCY-078 SoC SCY-078 is an investigational drug.
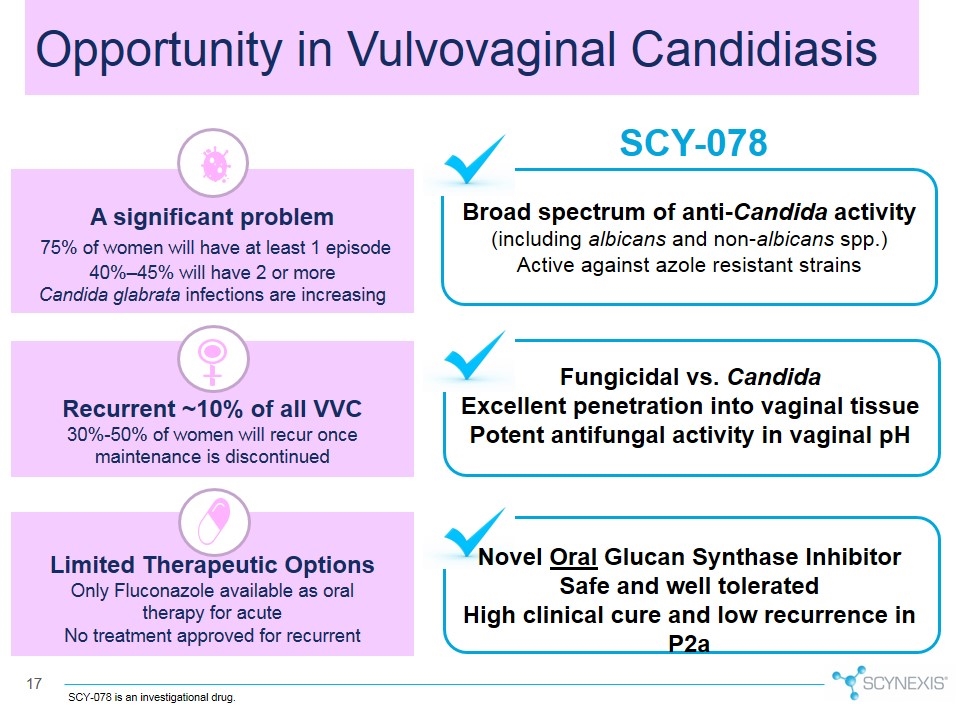
SCY-078 Broad spectrum of anti-Candida activity (including albicans and non-albicans spp.) Active against azole resistant strains Fungicidal vs. Candida Excellent penetration into vaginal tissue Potent antifungal activity in vaginal pH Novel Oral Glucan Synthase Inhibitor Safe and well tolerated High clinical cure and low recurrence in P2a A significant problem 75% of women will have at least 1 episode 40%–45% will have 2 or more Candida glabrata infections are increasing Limited Therapeutic Options Only Fluconazole available as oral therapy for acute No treatment approved for recurrent Recurrent ~10% of all VVC 30%-50% of women will recur once maintenance is discontinued Opportunity in Vulvovaginal Candidiasis SCY-078 is an investigational drug.
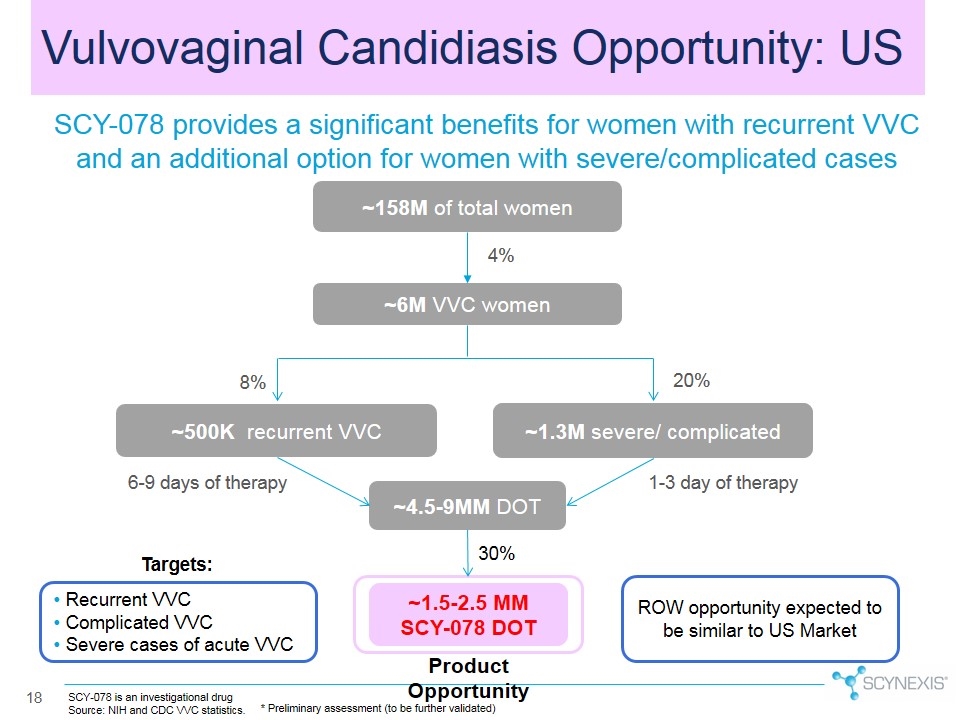
Vulvovaginal Candidiasis Opportunity: US SCY-078 is an investigational drug Source: NIH and CDC VVC statistics. 8% 20% 1-3 day of therapy 6-9 days of therapy ~158M of total women ~6M VVC women ~500K recurrent VVC ~4.5-9MM DOT ~1.3M severe/ complicated 4% ~1.5-2.5 MM SCY-078 DOT 30% SCY-078 provides a significant benefits for women with recurrent VVC and an additional option for women with severe/complicated cases Product Opportunity * Preliminary assessment (to be further validated) Recurrent VVC Complicated VVC Severe cases of acute VVC Targets: ROW opportunity expected to be similar to US Market
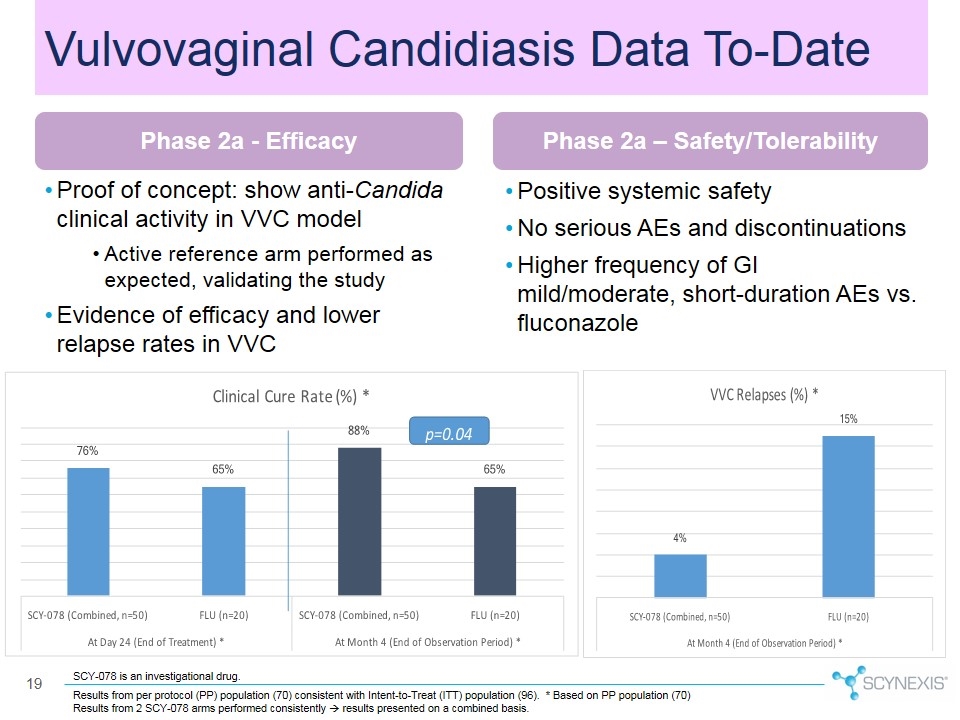
Proof of concept: show anti-Candida clinical activity in VVC model Active reference arm performed as expected, validating the study Evidence of efficacy and lower relapse rates in VVC Positive systemic safety No serious AEs and discontinuations Higher frequency of GI mild/moderate, short-duration AEs vs. fluconazole Results from per protocol (PP) population (70) consistent with Intent-to-Treat (ITT) population (96). * Based on PP population (70) Results from 2 SCY-078 arms performed consistently à results presented on a combined basis. Vulvovaginal Candidiasis Data To-Date Phase 2a - Efficacy Phase 2a – Safety/Tolerability SCY-078 is an investigational drug.
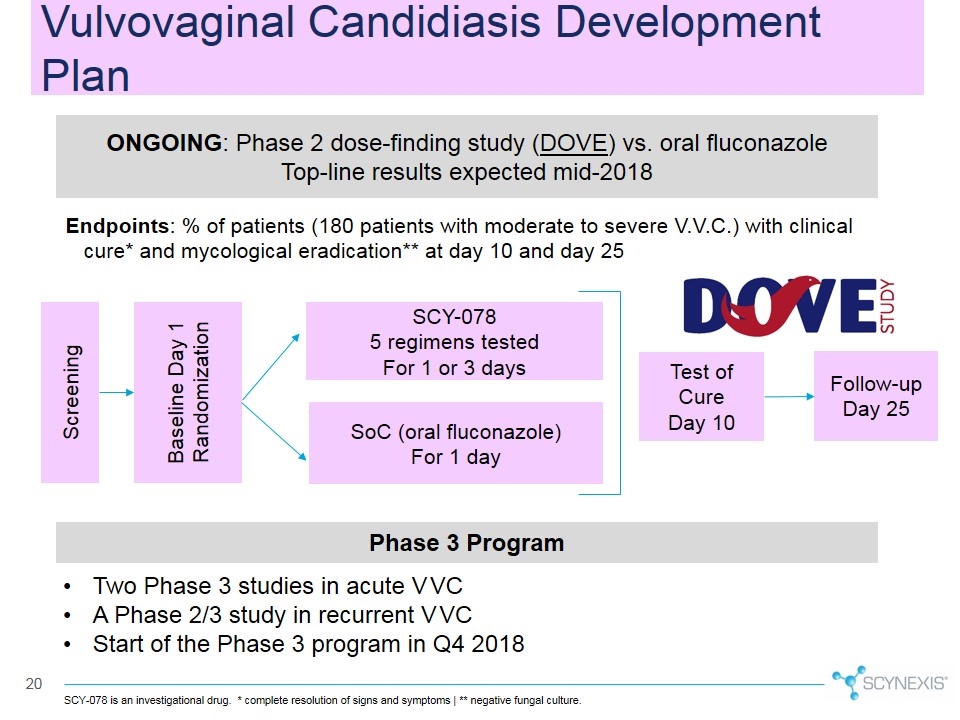
Vulvovaginal Candidiasis Development Plan SCY-078 is an investigational drug. * complete resolution of signs and symptoms | ** negative fungal culture. ONGOING: Phase 2 dose-finding study (DOVE) vs. oral fluconazole Top-line results expected mid-2018 Baseline Day 1 Randomization Screening SCY-078 5 regimens tested For 1 or 3 days SoC (oral fluconazole) For 1 day Test of Cure Day 10 Follow-up Day 25 Endpoints: % of patients (180 patients with moderate to severe V.V.C.) with clinical cure* and mycological eradication** at day 10 and day 25 Phase 3 Program Two Phase 3 studies in acute VVC A Phase 2/3 study in recurrent VVC Start of the Phase 3 program in Q4 2018
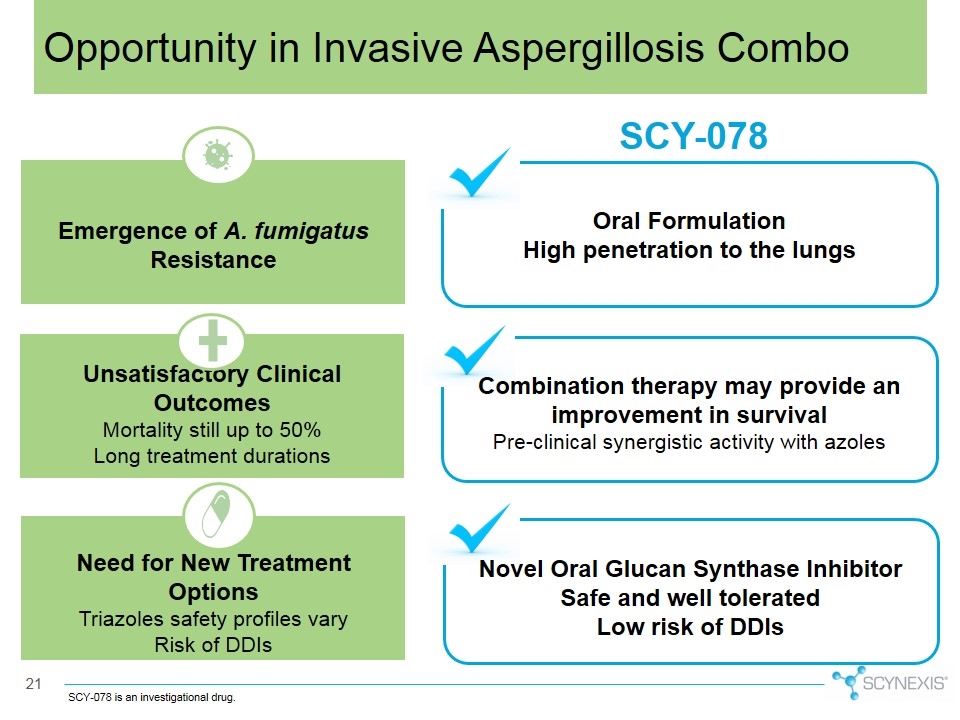
SCY-078 Oral Formulation High penetration to the lungs Combination therapy may provide an improvement in survival Pre-clinical synergistic activity with azoles Novel Oral Glucan Synthase Inhibitor Safe and well tolerated Low risk of DDIs Emergence of A. fumigatus Resistance Need for New Treatment Options Triazoles safety profiles vary Risk of DDIs Unsatisfactory Clinical Outcomes Mortality still up to 50% Long treatment durations Opportunity in Invasive Aspergillosis Combo SCY-078 is an investigational drug.
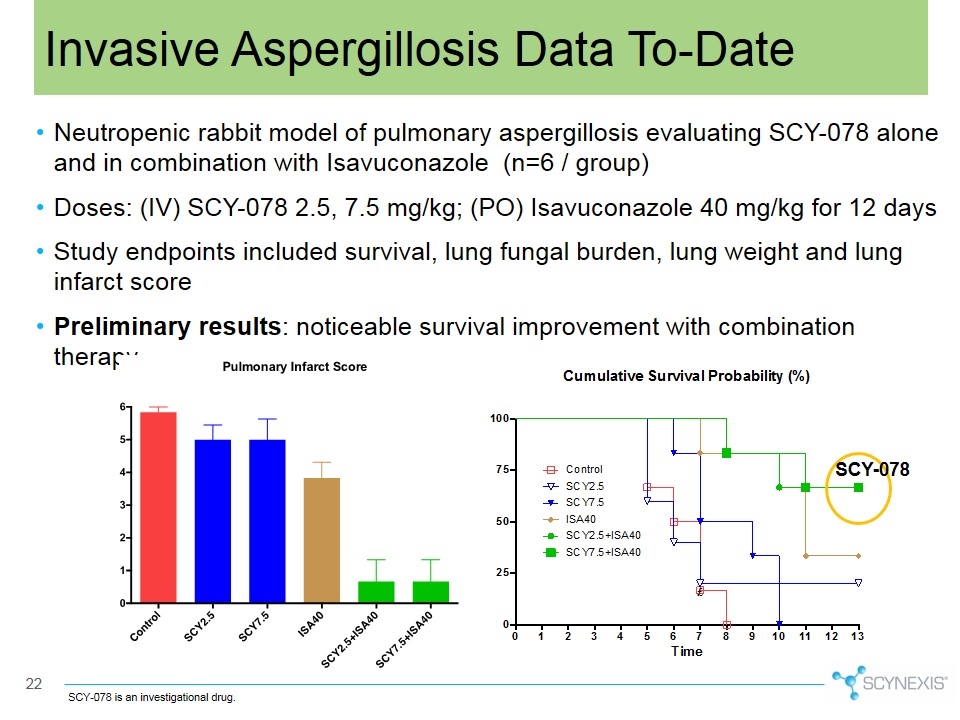
Neutropenic rabbit model of pulmonary aspergillosis evaluating SCY-078 alone and in combination with Isavuconazole (n=6 / group) Doses: (IV) SCY-078 2.5, 7.5 mg/kg; (PO) Isavuconazole 40 mg/kg for 12 days Study endpoints included survival, lung fungal burden, lung weight and lung infarct score Preliminary results: noticeable survival improvement with combination therapy Invasive Aspergillosis Data To-Date SCY-078 is an investigational drug. SCY-078
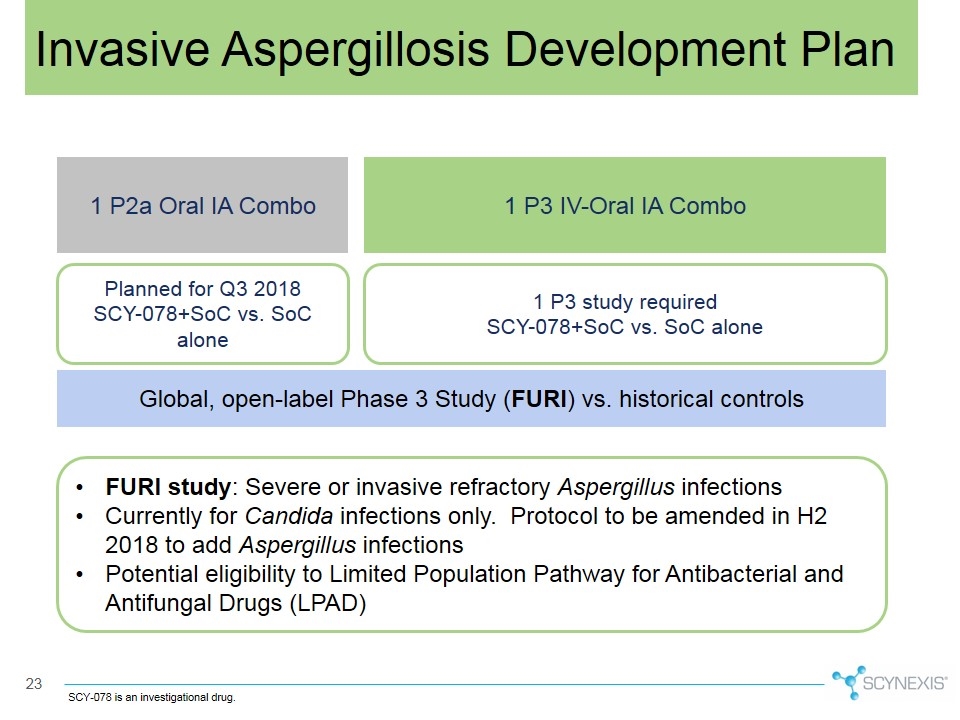
Invasive Aspergillosis Development Plan 1 P3 IV-Oral IA Combo 1 P2a Oral IA Combo Planned for Q3 2018 SCY-078+SoC vs. SoC alone 1 P3 study required SCY-078+SoC vs. SoC alone Global, open-label Phase 3 Study (FURI) vs. historical controls FURI study: Severe or invasive refractory Aspergillus infections Currently for Candida infections only. Protocol to be amended in H2 2018 to add Aspergillus infections Potential eligibility to Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) SCY-078 is an investigational drug.
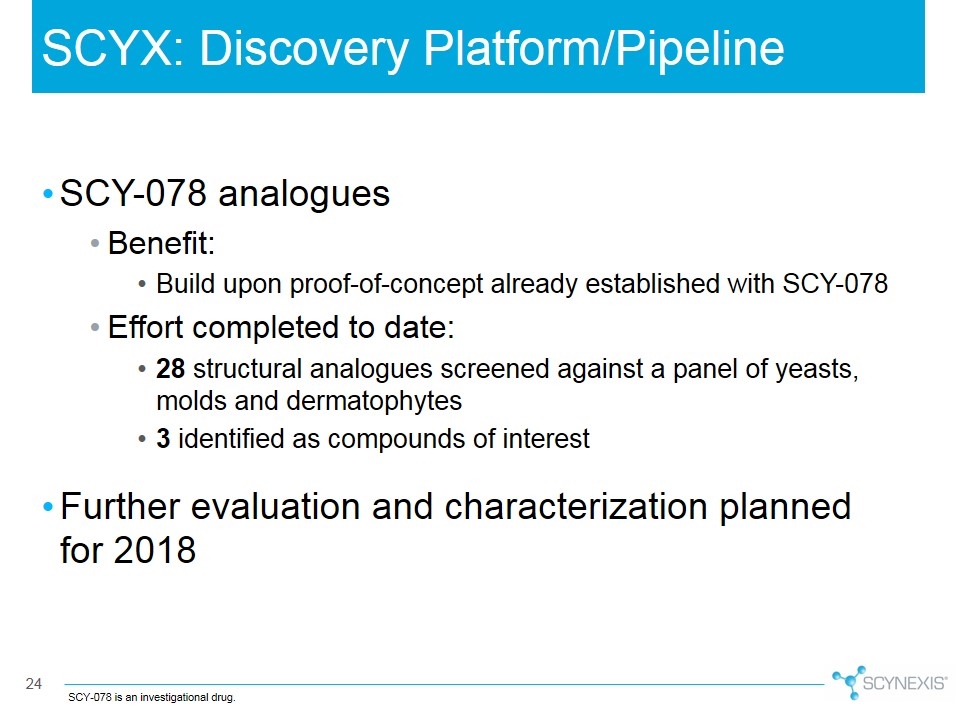
SCY-078 analogues Benefit: Build upon proof-of-concept already established with SCY-078 Effort completed to date: 28 structural analogues screened against a panel of yeasts, molds and dermatophytes 3 identified as compounds of interest Further evaluation and characterization planned for 2018 SCYX: Discovery Platform/Pipeline SCY-078 is an investigational drug.
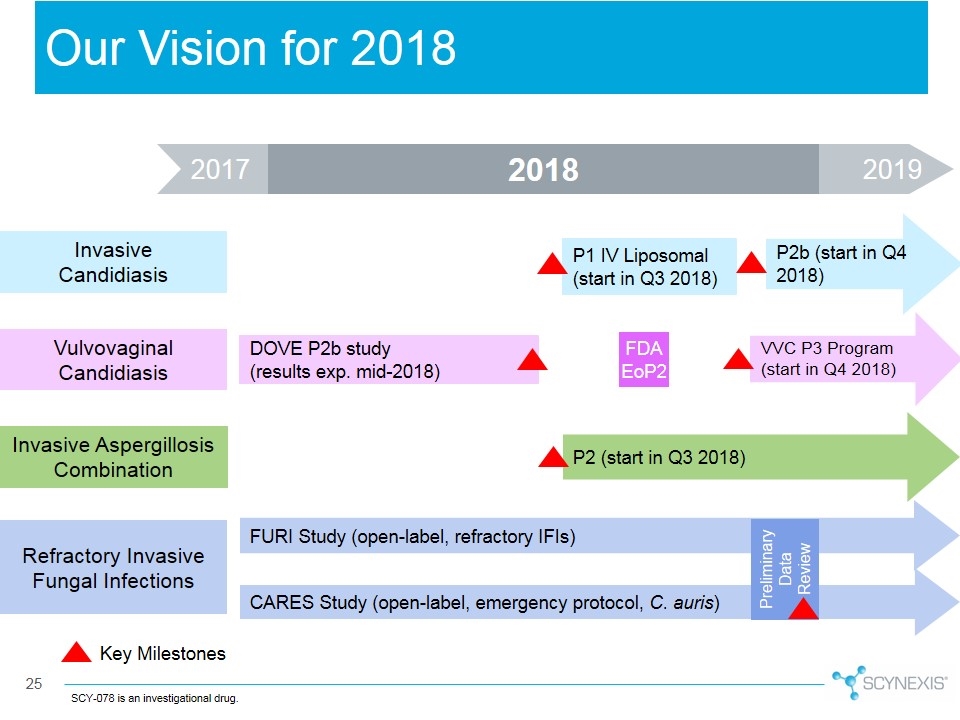
2017 Our Vision for 2018 2019 SCY-078 is an investigational drug. Vulvovaginal Candidiasis Invasive Aspergillosis Combination Refractory Invasive Fungal Infections CARES Study (open-label, emergency protocol, C. auris) P2 (start in Q3 2018) P2b (start in Q4 2018) DOVE P2b study (results exp. mid-2018) FURI Study (open-label, refractory IFIs) Invasive Candidiasis P1 IV Liposomal (start in Q3 2018) FDA EoP2 Preliminary Data Review VVC P3 Program (start in Q4 2018) Key Milestones 2018
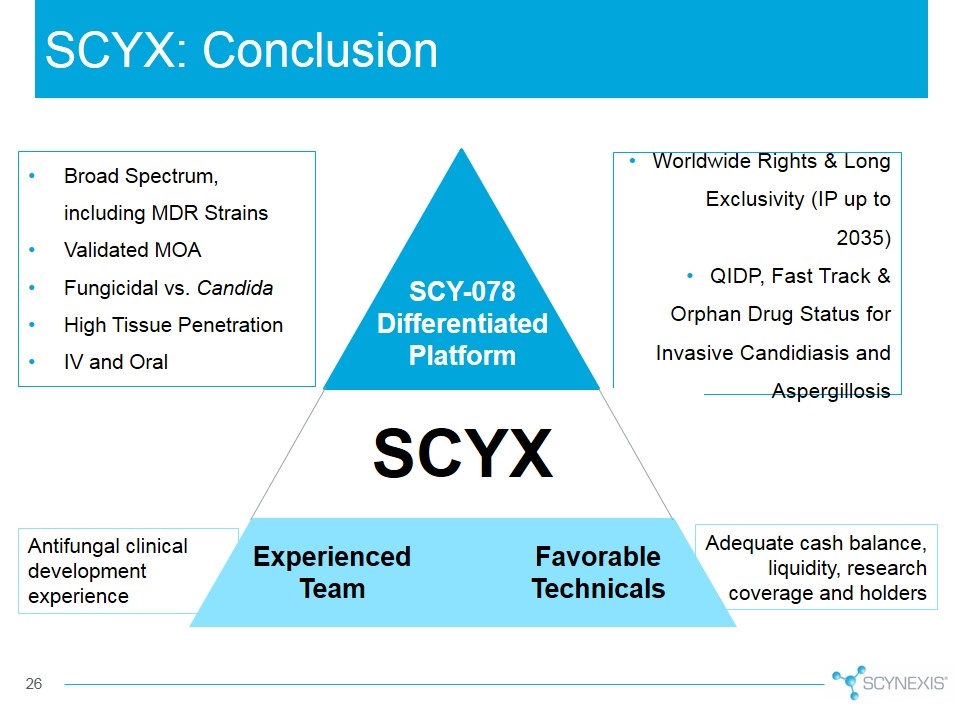
SCYX: Conclusion Adequate cash balance, liquidity, research coverage and holders Worldwide Rights & Long Exclusivity (IP up to 2035) QIDP, Fast Track & Orphan Drug Status for Invasive Candidiasis and Aspergillosis Broad Spectrum, including MDR Strains Validated MOA Fungicidal vs. Candida High Tissue Penetration IV and Oral SCYX Experienced Team Favorable Technicals SCY-078 Differentiated Platform Antifungal clinical development experience

Thank You SCY-078 – First of a Novel Oral/IV Triterpenoid Antifungal Family