
Investor Presentation January 2016 A New Path for Antifungal Treatments

1 Certain statements regarding SCYNEXIS, Inc. (the “Company”) made in this presentation constitute forward-looking statements, including, but not limited to, statements regarding our business strategies and goals, plans and prospects, market size, adoption rate, potential revenue, clinical validity and utility, growth opportunities, future products and product pipeline. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from our expectations. These risk and uncertainties include, but are not limited to: our ability to successfully develop SCY-078, including our ability to obtain FDA approval for SCY-078; the expected costs of studies and when they will begin; and our reliance on third parties to conduct our clinical studies. Forward-looking statements may be identified by the use of the words “anticipates,” “expects,” “intends,” “plans,” “could,” “should,” “would,” “may,” “will,” “believes,” “estimates,” “potential,” or “continue” and variations or similar expressions. These statements are based upon the current expectations and beliefs of management and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include, but are not limited to, risks and uncertainties discussed in the Company's most recent reports filed with the Securities and Exchange Commission ("SEC") including under the caption “Risks Factors” in the Company’s quarterly report on Form 10-Q or annual report on Form 10-K, which factors are incorporated herein by reference. Readers are cautioned not to place undue reliance on any of these forward-looking statements. The Company undertakes no obligation to update any of these forward-looking statements to reflect events or circumstances after the date of this presentation, or to reflect actual outcomes. Forward-looking Statements
SCYX: A New Path for Antifungal Treatments 2 Mission • Developing and commercializing innovative anti-infectives Objective • Moving beyond current antifungal therapies to address unmet needs Status • SCY-078: Introducing a new class of antifungals with a novel combination of attributes

SCYX: Highlights - SCY-078, a new antifungal class with a novel combination of attributes: • Validated mechanism of action • Both IV and oral formulations • Activity against Azole- and Echinocandin-resistant strains • Targeting multiple indications • Data from two Phase 2 studies expected in 2Q 2016 • Extended market exclusivity and IP protection - Significant unmet medical needs with global market potential - Favorable regulatory environment • Granted QIDP and Fast Track status - Experienced management team - Expected sufficient funds into 1H 2017 - $53.8mm in cash (as of 9/30/15) 3
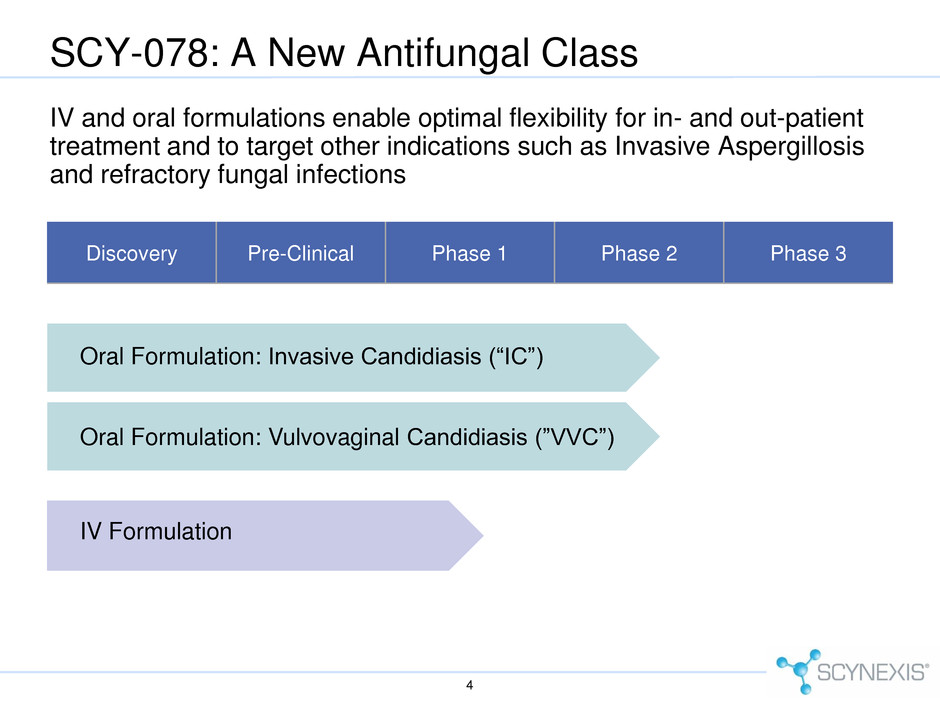
SCY-078: A New Antifungal Class IV and oral formulations enable optimal flexibility for in- and out-patient treatment and to target other indications such as Invasive Aspergillosis and refractory fungal infections Discovery Pre-Clinical Phase 1 Phase 2 Phase 3 Oral Formulation: Invasive Candidiasis (“IC”) Oral Formulation: Vulvovaginal Candidiasis (”VVC”) IV Formulation 4
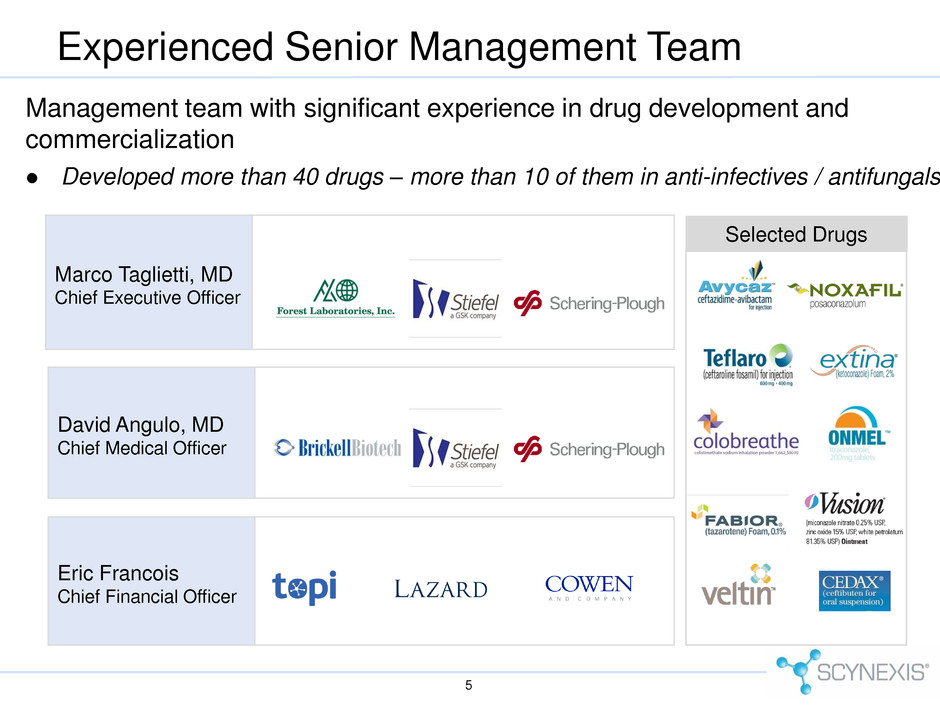
Experienced Senior Management Team 5 Management team with significant experience in drug development and commercialization Developed more than 40 drugs – more than 10 of them in anti-infectives / antifungals Marco Taglietti, MD Chief Executive Officer Eric Francois Chief Financial Officer David Angulo, MD Chief Medical Officer Selected Drugs
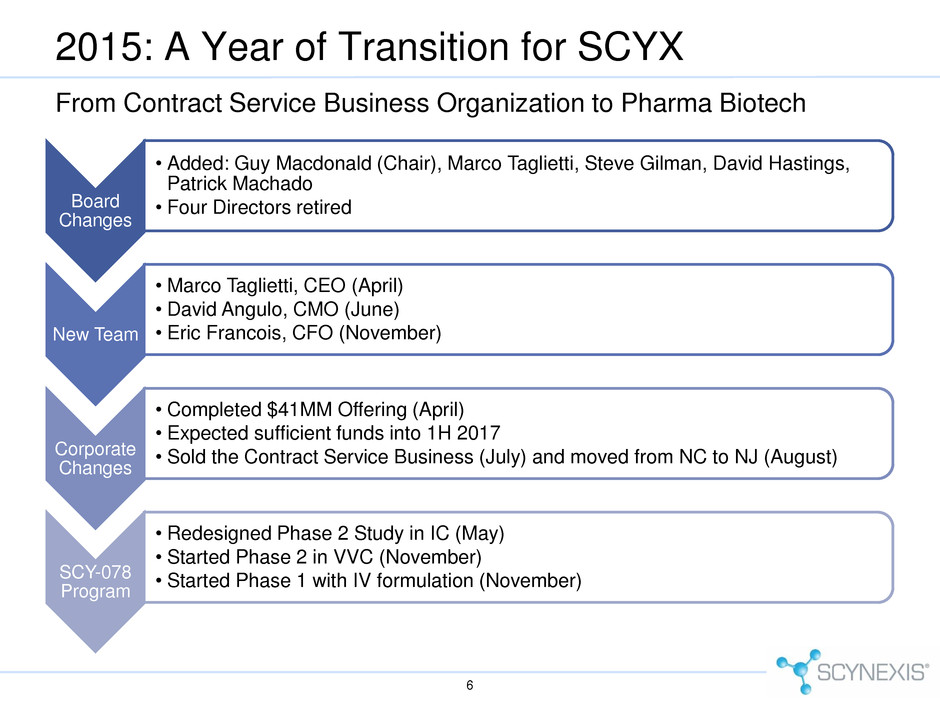
Board Changes • Added: Guy Macdonald (Chair), Marco Taglietti, Steve Gilman, David Hastings, Patrick Machado • Four Directors retired New Team • Marco Taglietti, CEO (April) • David Angulo, CMO (June) • Eric Francois, CFO (November) Corporate Changes • Completed $41MM Offering (April) • Expected sufficient funds into 1H 2017 • Sold the Contract Service Business (July) and moved from NC to NJ (August) SCY-078 Program • Redesigned Phase 2 Study in IC (May) • Started Phase 2 in VVC (November) • Started Phase 1 with IV formulation (November) 2015: A Year of Transition for SCYX 6 From Contract Service Business Organization to Pharma Biotech
7 Favorable Environment for Anti-infectives - Positive impact of GAIN Act (July 2012) and pending legislation (DISARM, ADAPT, 21st Century Cures): 1. Faster development and approval process 2. Increased access to regulatory agencies 3. Additional 5 years of market exclusivity 4. Potential for better reimbursement - Developing anti-infectives is typically less risky than other therapeutics • Pathogen is an external target • In vitro and in vivo models are highly predictive • Well-established PK/PD models - β-(1-3)-glucan synthase inhibitors recommended for 1st line treatment of invasive candidiasis in EU/US Clinical Guidelines • Moving away from Azoles in Invasive Candidiasis
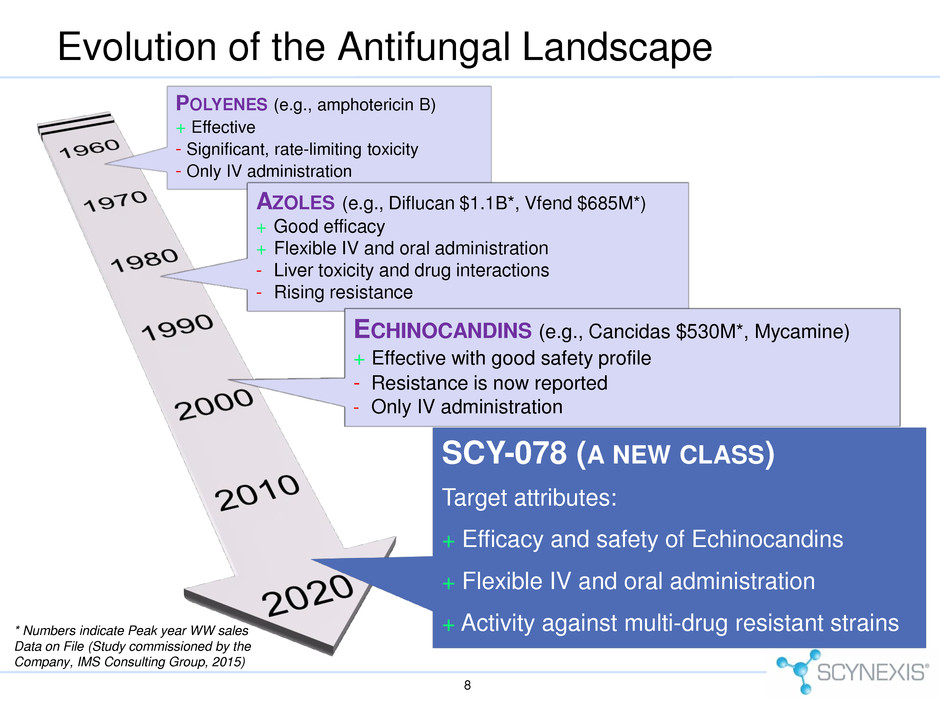
Evolution of the Antifungal Landscape 8 * Numbers indicate Peak year WW sales Data on File (Study commissioned by the Company, IMS Consulting Group, 2015) POLYENES (e.g., amphotericin B) + Effective - Significant, rate-limiting toxicity - Only IV administration AZOLES (e.g., Diflucan $1.1B*, Vfend $685M*) + Good efficacy + Flexible IV and oral administration - Liver toxicity and drug interactions - Rising resistance ECHINOCANDINS (e.g., Cancidas $530M*, Mycamine) + Effective with good safety profile - Resistance is now reported - Only IV administration SCY-078 (A NEW CLASS) Target attributes: + Efficacy and safety of Echinocandins + Flexible IV and oral administration + Activity against multi-drug resistant strains
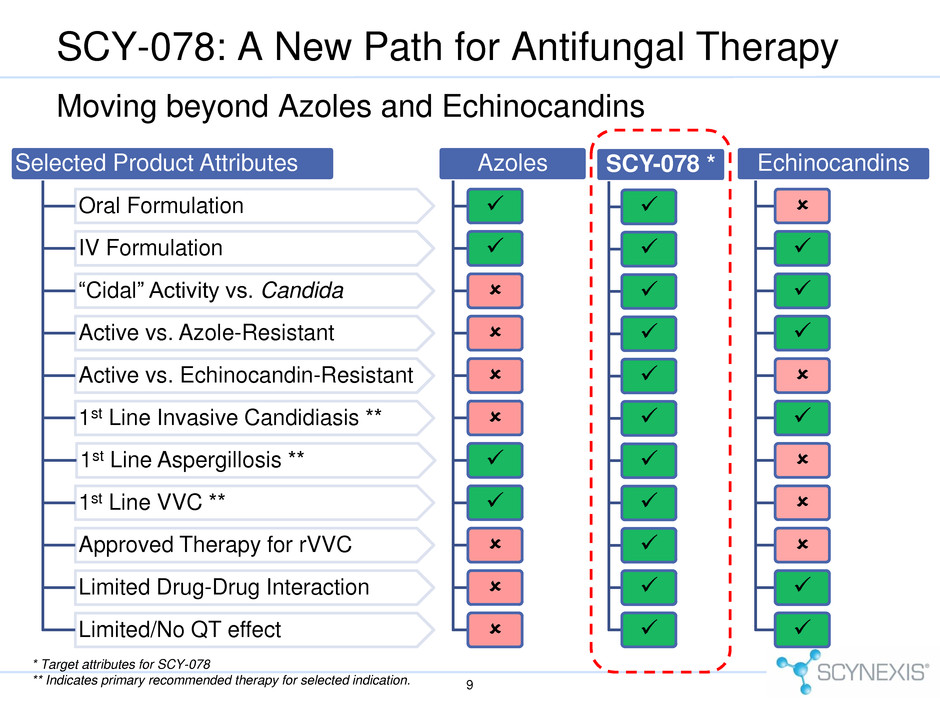
SCY-078: A New Path for Antifungal Therapy 9 Selected Product Attributes Oral Formulation IV Formulation “Cidal” Activity vs. Candida Active vs. Azole-Resistant Active vs. Echinocandin-Resistant 1st Line Invasive Candidiasis ** 1st Line Aspergillosis ** 1st Line VVC ** Approved Therapy for rVVC Limited Drug-Drug Interaction Limited/No QT effect Azoles Echinocandins SCY-078 * Moving beyond Azoles and Echinocandins * Target attributes for SCY-078 ** Indicates primary recommended therapy for selected indication.
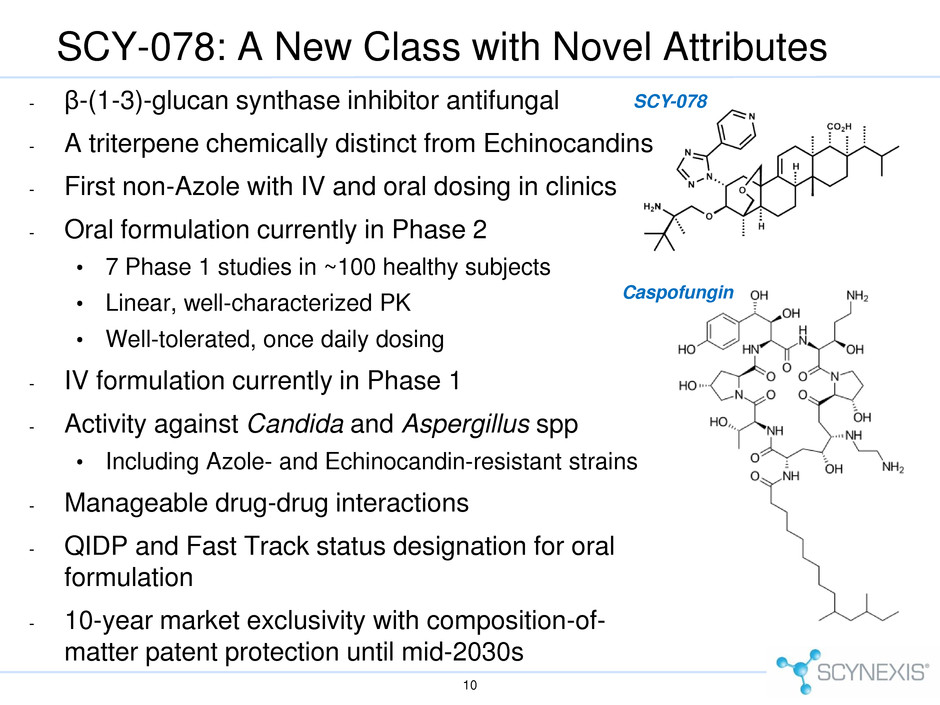
SCY-078: A New Class with Novel Attributes - β-(1-3)-glucan synthase inhibitor antifungal - A triterpene chemically distinct from Echinocandins - First non-Azole with IV and oral dosing in clinics - Oral formulation currently in Phase 2 • 7 Phase 1 studies in ~100 healthy subjects • Linear, well-characterized PK • Well-tolerated, once daily dosing - IV formulation currently in Phase 1 - Activity against Candida and Aspergillus spp • Including Azole- and Echinocandin-resistant strains - Manageable drug-drug interactions - QIDP and Fast Track status designation for oral formulation - 10-year market exclusivity with composition-of- matter patent protection until mid-2030s 10 SCY-078 Caspofungin
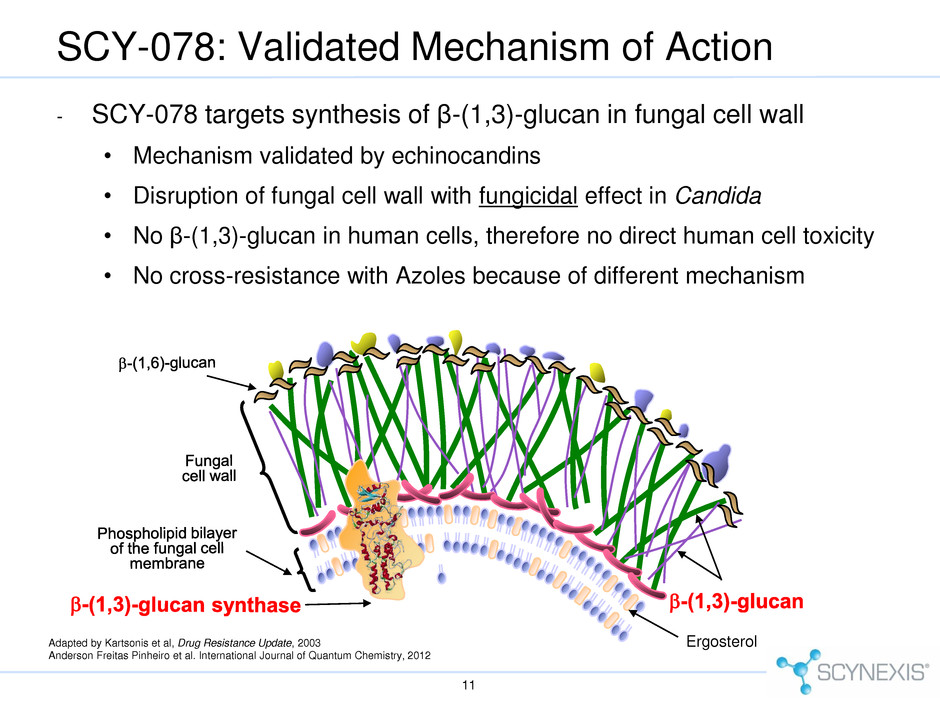
Ergosterol SCY-078: Validated Mechanism of Action 11 Adapted by Kartsonis et al, Drug Resistance Update, 2003 Anderson Freitas Pinheiro et al. International Journal of Quantum Chemistry, 2012 - SCY-078 targets synthesis of β-(1,3)-glucan in fungal cell wall • Mechanism validated by echinocandins • Disruption of fungal cell wall with fungicidal effect in Candida • No β-(1,3)-glucan in human cells, therefore no direct human cell toxicity • No cross-resistance with Azoles because of different mechanism
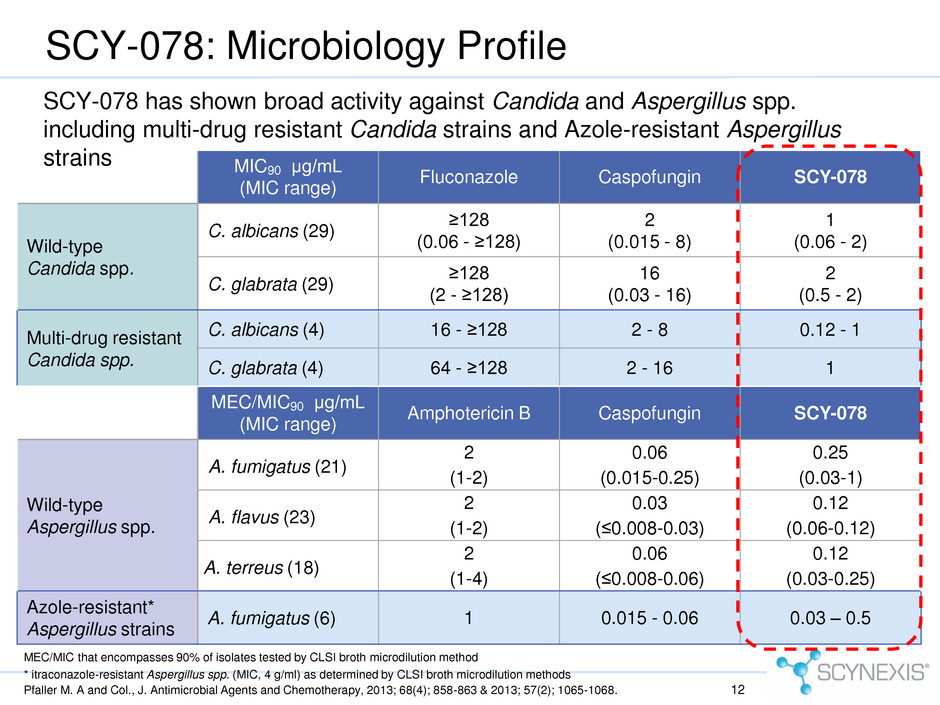
SCY-078: Microbiology Profile * itraconazole-resistant Aspergillus spp. (MIC, 4 g/ml) as determined by CLSI broth microdilution methods Pfaller M. A and Col., J. Antimicrobial Agents and Chemotherapy, 2013; 68(4); 858-863 & 2013; 57(2); 1065-1068. MIC90 μg/mL (MIC range) Fluconazole Caspofungin SCY-078 Wild-type Candida spp. C. albicans (29) ≥128 (0.06 - ≥128) 2 (0.015 - 8) 1 (0.06 - 2) C. glabrata (29) ≥128 (2 - ≥128) 16 (0.03 - 16) 2 (0.5 - 2) Multi-drug resistant Candida spp. C. albicans (4) 16 - ≥128 2 - 8 0.12 - 1 C. glabrata (4) 64 - ≥128 2 - 16 1 SCY-078 has shown broad activity against Candida and Aspergillus spp. including multi-drug resistant Candida strains and Azole-resistant Aspergillus strains MEC/MIC90 μg/mL (MIC range) Amphotericin B Caspofungin SCY-078 Wild-type Aspergillus spp. A. fumigatus (21) 2 (1-2) 0.06 (0.015-0.25) 0.25 (0.03-1) A. flavus (23) 2 (1-2) 0.03 (≤0.008-0.03) 0.12 (0.06-0.12) A. terreus (18) 2 (1-4) 0.06 (≤0.008-0.06) 0.12 (0.03-0.25) Azole-resistant* Aspergillus strains A. fumigatus (6) 1 0.015 - 0.06 0.03 – 0.5 MEC/MIC that encompasses 90% of isolates tested by CLSI broth microdilution method 12
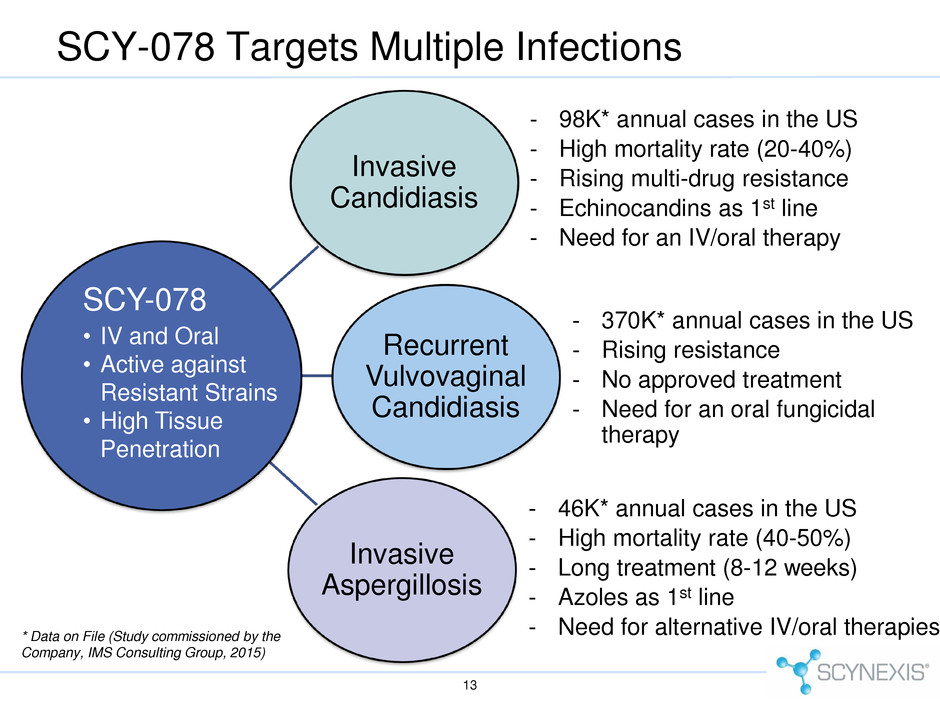
SCY-078 Targets Multiple Infections 13 Invasive Aspergillosis - 46K* annual cases in the US - High mortality rate (40-50%) - Long treatment (8-12 weeks) - Azoles as 1st line - Need for alternative IV/oral therapies Invasive Candidiasis - 98K* annual cases in the US - High mortality rate (20-40%) - Rising multi-drug resistance - Echinocandins as 1st line - Need for an IV/oral therapy Recurrent Vulvovaginal Candidiasis - 370K* annual cases in the US - Rising resistance - No approved treatment - Need for an oral fungicidal therapy * Data on File (Study commissioned by the Company, IMS Consulting Group, 2015) SCY-078 • IV and Oral • Active against Resistant Strains • High Tissue Penetration
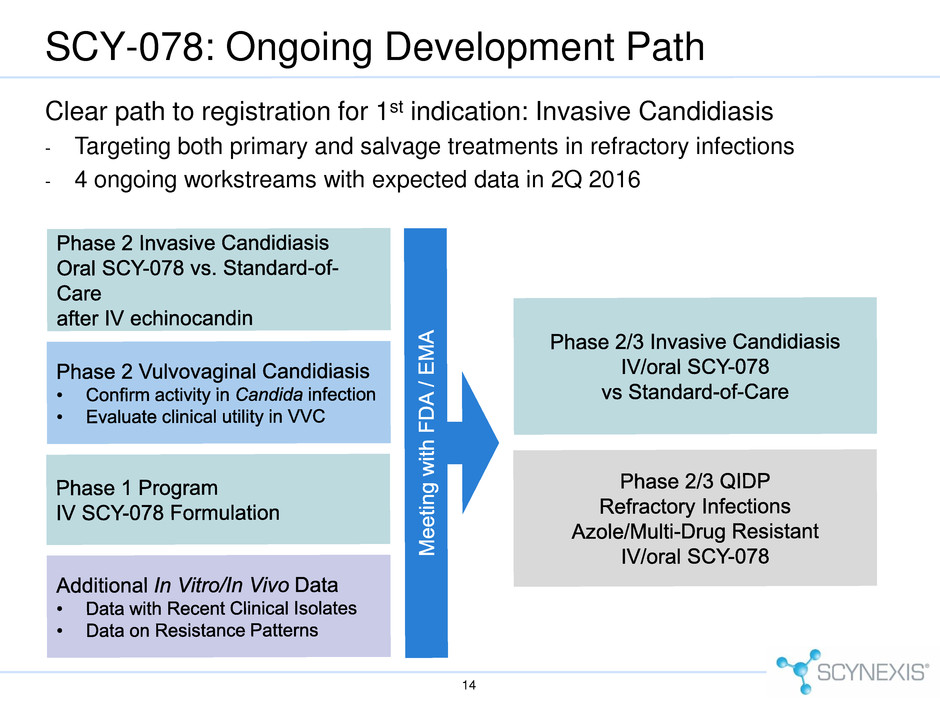
SCY-078: Ongoing Development Path 14 Clear path to registration for 1st indication: Invasive Candidiasis - Targeting both primary and salvage treatments in refractory infections - 4 ongoing workstreams with expected data in 2Q 2016
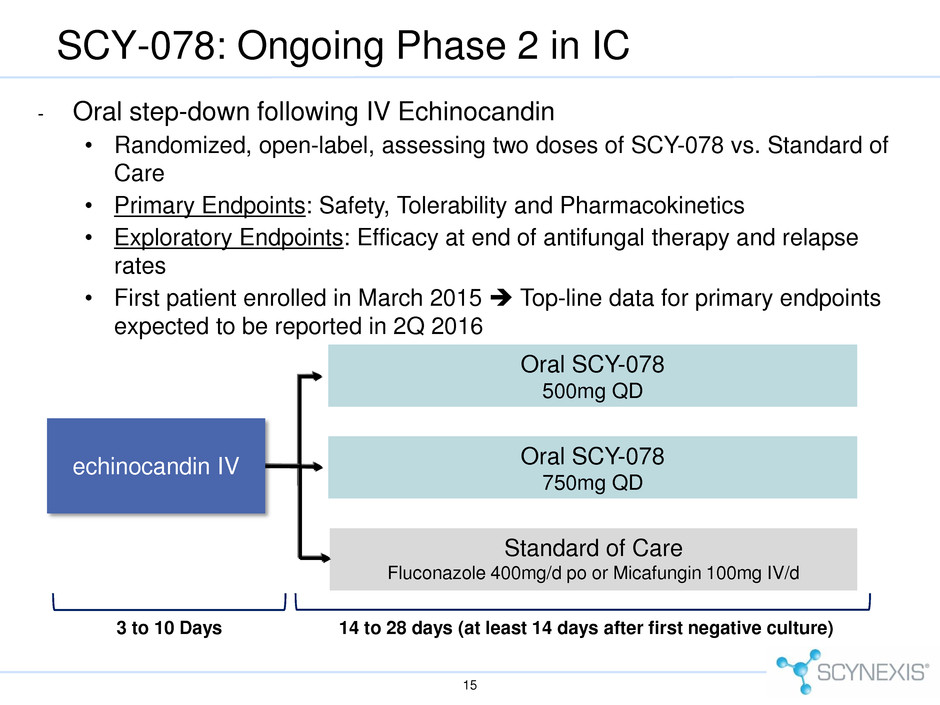
- Oral step-down following IV Echinocandin • Randomized, open-label, assessing two doses of SCY-078 vs. Standard of Care • Primary Endpoints: Safety, Tolerability and Pharmacokinetics • Exploratory Endpoints: Efficacy at end of antifungal therapy and relapse rates • First patient enrolled in March 2015 Top-line data for primary endpoints expected to be reported in 2Q 2016 15 SCY-078: Ongoing Phase 2 in IC 14 to 28 days (at least 14 days after first negative culture) Oral SCY-078 500mg QD Oral SCY-078 750mg QD Standard of Care Fluconazole 400mg/d po or Micafungin 100mg IV/d 3 to 10 Days echinocandin IV
- Oral treatment for vulvovaginal candidiasis • Randomized, evaluator-blinded study • Patients with moderate to severe VVC • Two dose-regimens of oral SCY-078 (5 or 3 days) compared to standard dose-regimen of Fluconazole • Primary Endpoints: Safety and Efficacy (clinical & microbiological outcome) at Day 24 • Secondary / Exploratory Endpoints: Relapse rates, clinical & microbiological outcomes up to Month 4 • First patient enrolled in Nov. 2015 Top-line data for primary endpoints expected to be reported in 2Q 2016 16 SCY-078: Ongoing Phase 2 in VVC
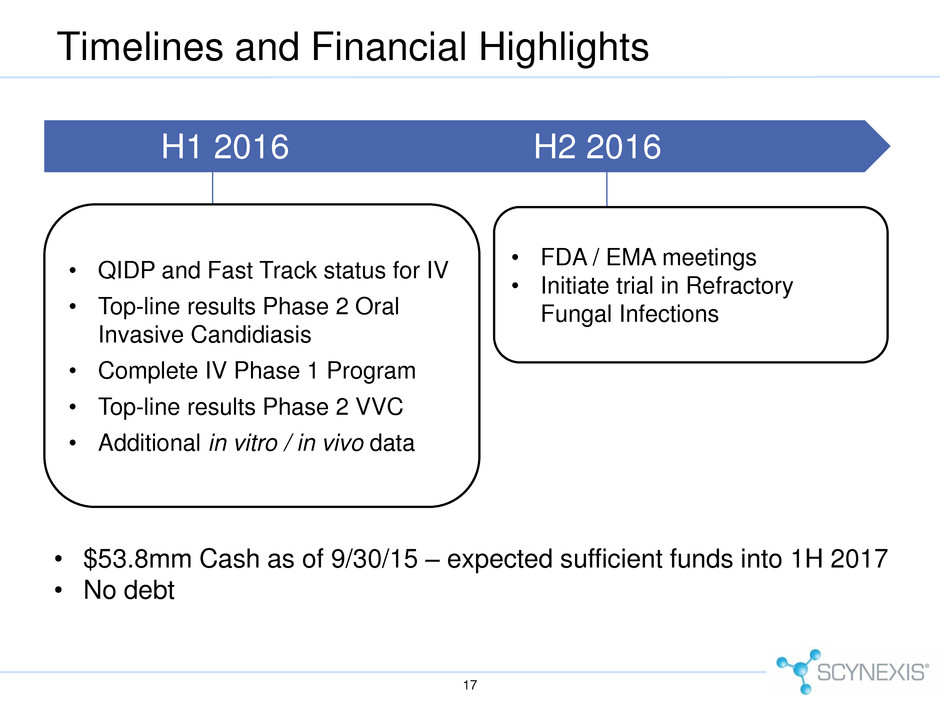
Timelines and Financial Highlights 17 H1 2016 H2 2016 • QIDP and Fast Track status for IV • Top-line results Phase 2 Oral Invasive Candidiasis • Complete IV Phase 1 Program • Top-line results Phase 2 VVC • Additional in vitro / in vivo data • $53.8mm Cash as of 9/30/15 – expected sufficient funds into 1H 2017 • No debt • FDA / EMA meetings • Initiate trial in Refractory Fungal Infections
SCYX: Highlights - SCY-078, a new antifungal class with a novel combination of attributes: • Validated mechanism of action • Both IV and oral formulations • Activity against Azole- and Echinocandin-resistant strains • Targeting multiple indications • Data from two Phase 2 studies expected in 2Q 2016 • Extended market exclusivity and IP protection - Significant unmet medical needs with global market potential - Favorable regulatory environment • Granted QIDP and Fast Track status - Experienced management team - Expected sufficient funds into 1H 2017 - $53.8mm in cash (as of 9/30/15) 18

NASDAQ: SCYX www.scynexis.com 19