
SCYNEXIS, Inc. January 2015

1 Certain statements regarding SCYNEXIS, Inc. (the “Company”) made in this presentation may constitute forward-looking statements, including, but not limited to, statements regarding our business strategies and goals, plans and prospects, market size, adoption rate, potential revenue, clinical validity and utility, growth opportunities, future products and product pipeline. Forward- looking statements are subject to risks and uncertainties that could cause actual results to differ materially from our expectations. These risk and uncertainties include but are not limited to our ability to successfully develop SCY-078, including an IV formulation of SCY-078; our expectations regarding QIDP designation; our ability to obtain FDA approval for SCY-078; the expected costs of studies and when they will begin and our reliance on third parties to conduct our clinical studies.. Forward-looking statements may be identified by the use of the words “anticipates,” “expects,” “intends,” “plans,” “could,” “should,” “would,” “may,” “will ,” “believes,” “estimates,” “potential,” or “continue” and variations or similar expressions. These statements are based upon the current expectations and beliefs of management and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include, but are not limited to, risks and uncertainties discussed in the company's most recent reports filed with the Securities and Exchange Commission ("SEC") and other risks and uncertainties detailed from time to time in the Company's filings with the SEC, which factors are incorporated herein by reference. Readers are cautioned not to place undue reliance on any of these forward - looking statements. The Company undertakes no obligation to update any of these forward-looking statements to reflect events or circumstances after the date of this presentation, or to reflect actual outcomes. Forward-looking Statements
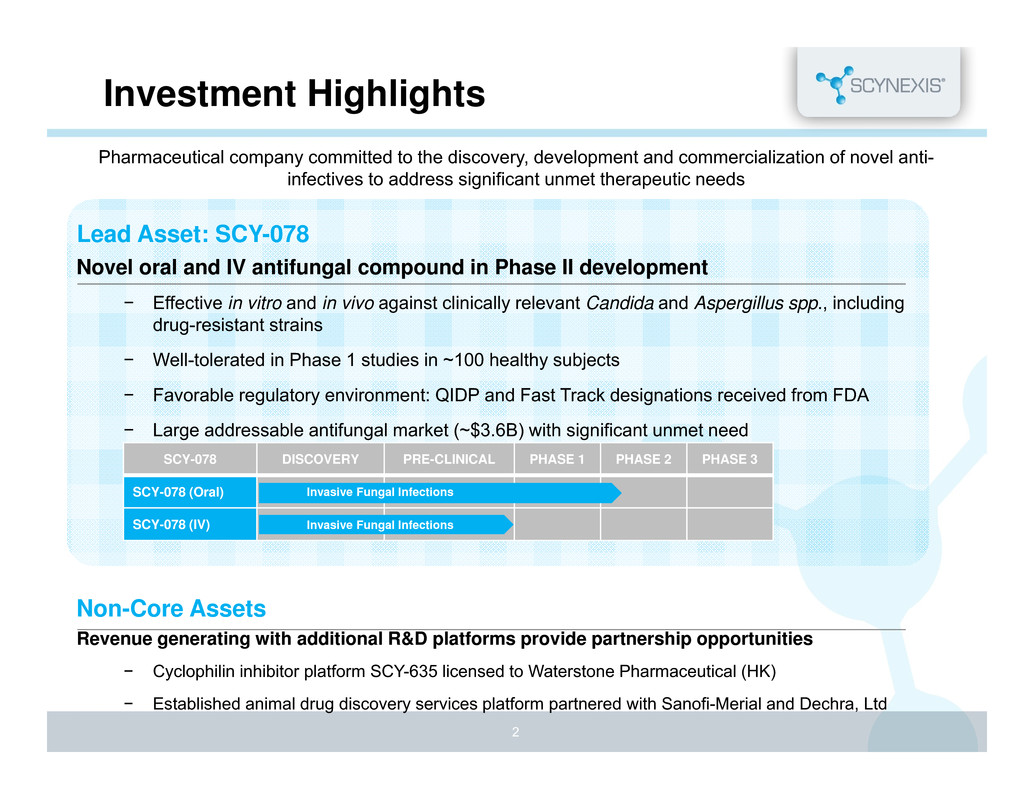
Investment Highlights Lead Asset: SCY-078 Novel oral and IV antifungal compound in Phase II development − Effective in vitro and in vivo against clinically relevant Candida and Aspergillus spp., including drug-resistant strains − Well-tolerated in Phase 1 studies in ~100 healthy subjects − Favorable regulatory environment: QIDP and Fast Track designations received from FDA − Large addressable antifungal market (~$3.6B) with significant unmet need Non-Core Assets Revenue generating with additional R&D platforms provide partnership opportunities − Cyclophilin inhibitor platform SCY-635 licensed to Waterstone Pharmaceutical (HK) − Established animal drug discovery services platform partnered with Sanofi-Merial and Dechra, Ltd 2 Pharmaceutical company committed to the discovery, development and commercialization of novel anti- infectives to address significant unmet therapeutic needs SCY-078 DISCOVERY PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 SCY-078 (Oral) SCY-078 (IV) Invasive Fungal Infections Invasive Fungal Infections
Invasive Fungal Infections Pose a Serious Clinical Problem Invasive candidiasis • Most common invasive fungal infection and 4th most common cause of hospital acquired blood stream infection in US • Mortality 25 to 40% despite treatment • Identified by CDC as an antimicrobial resistance threat in 2013 • Increasing prevalence of Candida spp. with high level of resistance to azoles and increasing prevalence of Multi Drug Resistant species (C. glabrata) • Limited therapeutic options for azole and echinocandin resistant Candida infections • Drug resistant Candida infections associated with higher mortality, hospital cost and longer length of stay • Treatment guidelines changing as result of resistance Invasive aspergillosis • Second most common invasive fungal infection • Mortality approaches 90% in the most severely immunocompromised Due to high mortality associated with a delay in treatment, ~2/3 of systemic antifungal use occurs before a diagnosis is confirmed 3
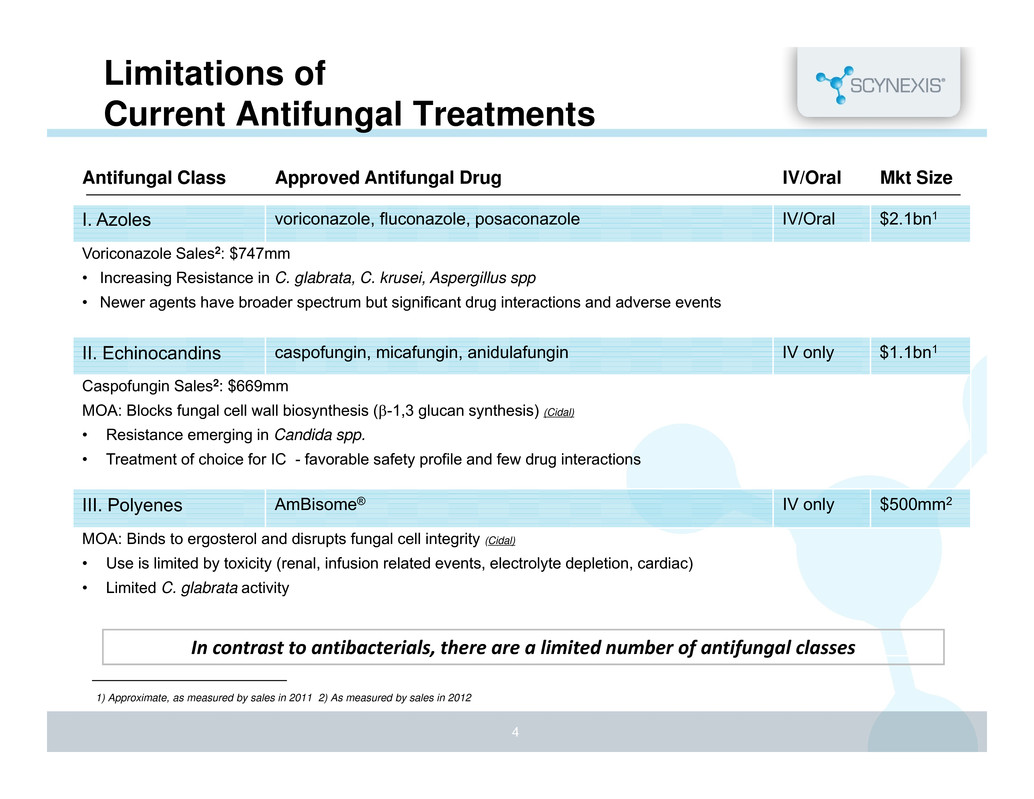
Limitations of Current Antifungal Treatments Antifungal Class Approved Antifungal Drug IV/Oral Mkt Size I. Azoles voriconazole, fluconazole, posaconazole IV/Oral $2.1bn1 Voriconazole Sales2: $747mm • Increasing Resistance in C. glabrata, C. krusei, Aspergillus spp • Newer agents have broader spectrum but significant drug interactions and adverse events II. Echinocandins caspofungin, micafungin, anidulafungin IV only $1.1bn1 Caspofungin Sales2: $669mm MOA: Blocks fungal cell wall biosynthesis (-1,3 glucan synthesis) (Cidal) • Resistance emerging in Candida spp. • Treatment of choice for IC - favorable safety profile and few drug interactions III. Polyenes AmBisome® IV only $500mm2 MOA: Binds to ergosterol and disrupts fungal cell integrity (Cidal) • Use is limited by toxicity (renal, infusion related events, electrolyte depletion, cardiac) • Limited C. glabrata activity 1) Approximate, as measured by sales in 2011 2) As measured by sales in 2012 4 In contrast to antibacterials, there are a limited number of antifungal classes
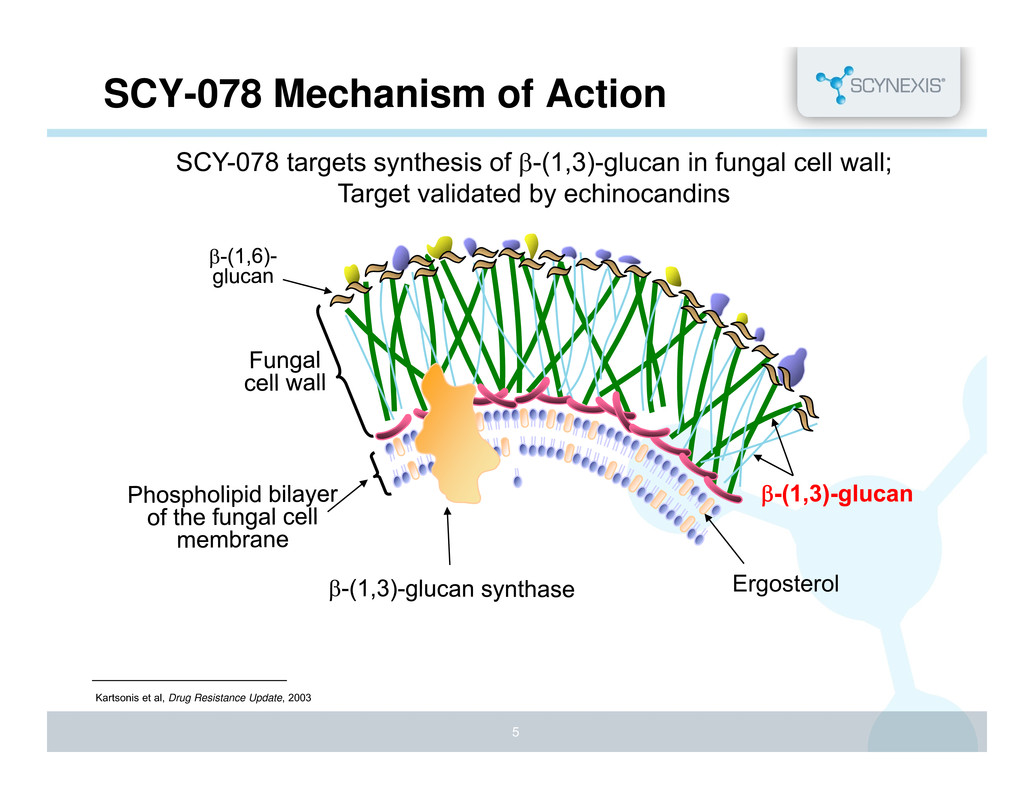
Ergosterol SCY-078 Mechanism of Action Kartsonis et al, Drug Resistance Update, 2003 5 SCY-078 targets synthesis of -(1,3)-glucan in fungal cell wall; Target validated by echinocandins
SCY-078 is a first-in-class enfumafungin antifungal − New chemical class issued from natural compound − Strong IP with long patent life − First non-azole with IV and oral formulations − IV formulation IND enabling studies underway, FIM 2H-15 − Oral formulation entering Phase 2 1H-2015 − Activity against Candida and Aspergillus spp., including azole- and echinocandin-resistant strains − Favorable safety and tolerability profile − Manageable drug-drug interactions SCY-078 Key Product Attributes 6
SCY-078 Significantly De-risked vs. Phase 2 Assets in Other Therapeutic Areas Development in anti-infectives is unusual − The target (a microbe) can be isolated − The drug works on the microbe, not the patient − Studies in a test tube (MICs) and in animal models reliably predict efficacy in man Unlike most other drugs… − Antibiotic blood levels, − The minimum inhibitory concentration (MIC) of the drug for the bug, and − Response have a predictable relationship: with rare exception the concentrations of drug in a mouse that are effective are also effective in man Still need other data, primarily safety, but PK/PD significantly de-risks concerns about efficacy failures 7
SCY-078: Well-tolerated in Phase 1 SCY-078 evaluated in seven Phase 1 studies in ~100 healthy subjects − Half life supports once daily dosing − Predicted human efficacious dose of ~ 500mg daily based on murine disseminated candidiasis PK/PD studies Favorable safety and tolerability profile − Generally safe and well tolerated at single doses up to 1600mg and multiple doses of 800mg/day for up to 28 days − Most common adverse events were gastrointestinal (nausea, diarrhea) − Majority mild to moderate and did not lead to discontinuation of therapy Metabolized primarily by glucuronidation and oxidative mechanisms involving CYP-3A4 8
GAIN Act − SCY-078 oral formulation granted QIDP designation by FDA for invasive candidiasis and invasive aspergillosis Fast Track − SCY-078 oral formulation granted Fast Track designation by FDA for invasive candidiasis and invasive aspergillosis DISARM Act (Introduced) − Allowing value based pricing for antimicrobial products ADAPT Act (Pending) − Allow FDA to promptly approve drugs for targeted and limited patient populations Impact of Current and Pending Legislation − Increased awareness of urgent need for new antifungals − Additional market exclusivity − Possibility for faster development pathway − Potential for pricing power in resistant patient populations Favorable Regulatory Environment 6
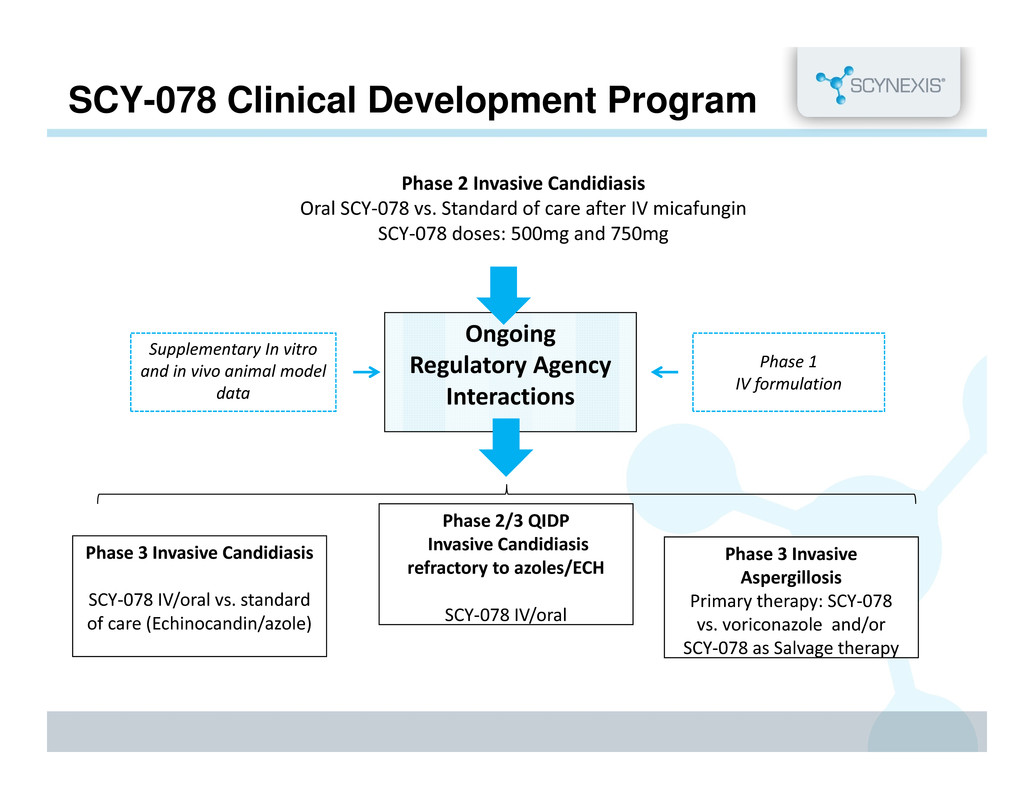
SCY-078 Clinical Development Program Phase 1 IV formulation Ongoing Regulatory Agency Interactions Phase 3 Invasive Aspergillosis Primary therapy: SCY‐078 vs. voriconazole and/or SCY‐078 as Salvage therapy Phase 2 Invasive Candidiasis Oral SCY‐078 vs. Standard of care after IV micafungin SCY‐078 doses: 500mg and 750mg Supplementary In vitro and in vivo animal model data Phase 2/3 QIDP Invasive Candidiasis refractory to azoles/ECH SCY‐078 IV/oral Phase 3 Invasive Candidiasis SCY‐078 IV/oral vs. standard of care (Echinocandin/azole)
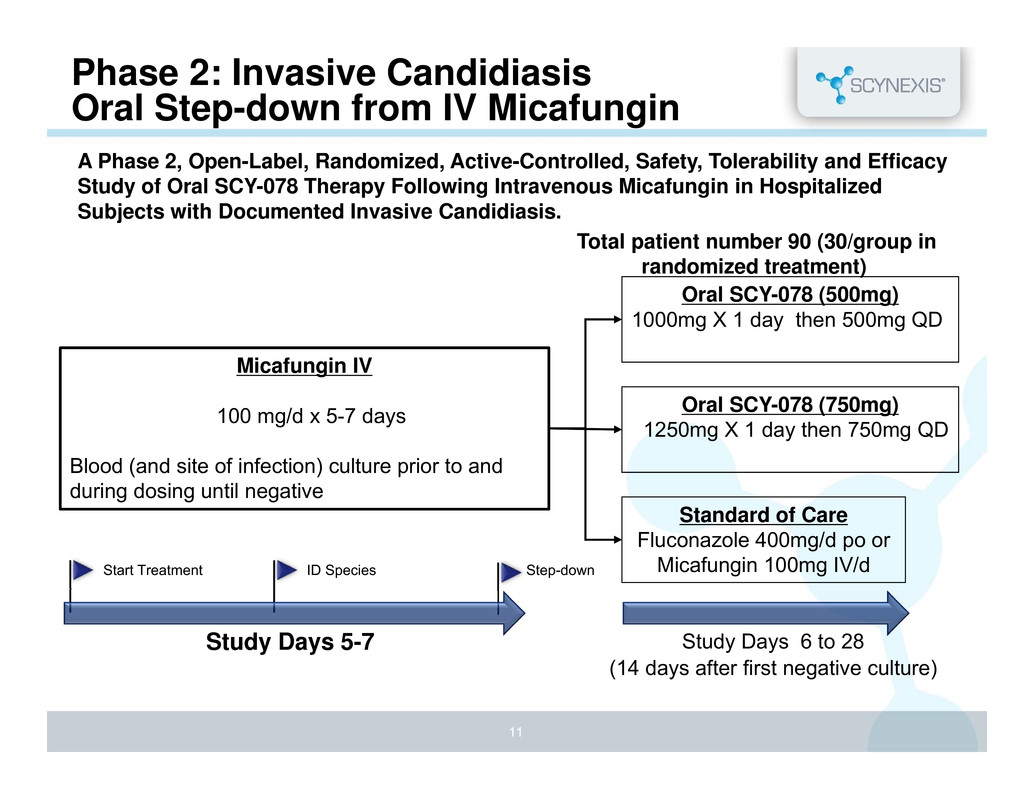
Phase 2: Invasive Candidiasis Oral Step-down from IV Micafungin Micafungin IV 100 mg/d x 5-7 days Blood (and site of infection) culture prior to and during dosing until negative Study Days 5-7 Study Days 6 to 28 (14 days after first negative culture) Oral SCY-078 (500mg) 1000mg X 1 day then 500mg QD Oral SCY-078 (750mg) 1250mg X 1 day then 750mg QD Standard of Care Fluconazole 400mg/d po or Micafungin 100mg IV/d A Phase 2, Open-Label, Randomized, Active-Controlled, Safety, Tolerability and Efficacy Study of Oral SCY-078 Therapy Following Intravenous Micafungin in Hospitalized Subjects with Documented Invasive Candidiasis. Total patient number 90 (30/group in randomized treatment) Start Treatment ID Species Step-down 11
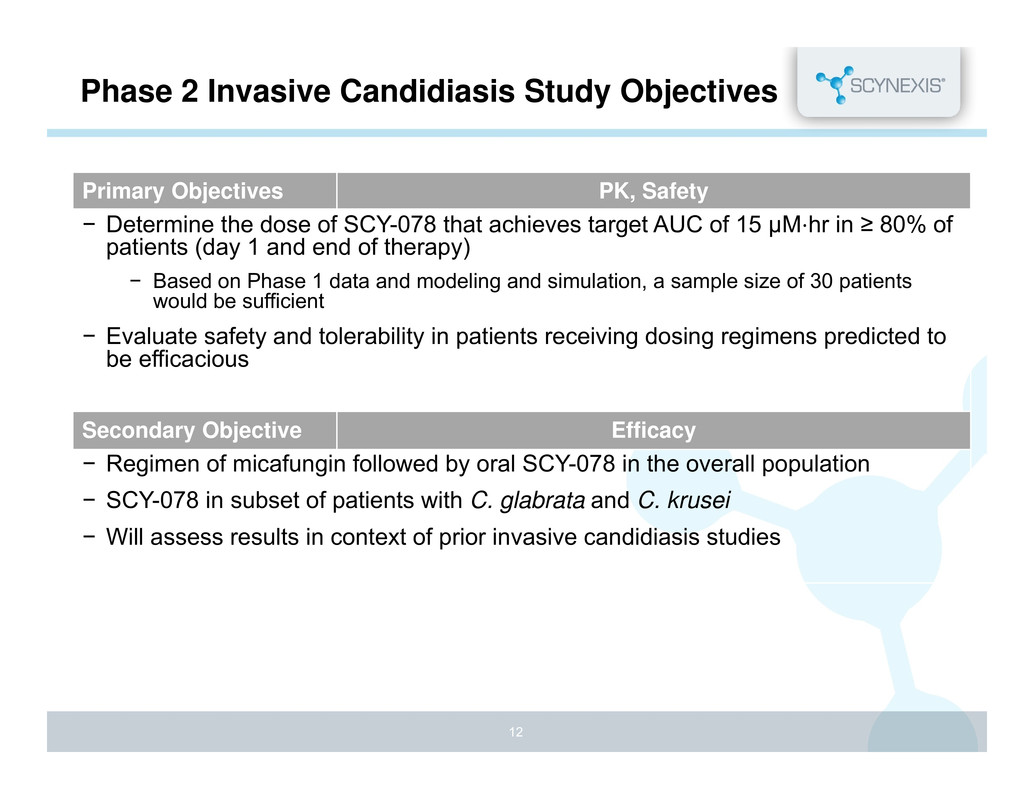
Phase 2 Invasive Candidiasis Study Objectives Primary Objectives PK, Safety − Determine the dose of SCY-078 that achieves target AUC of 15 μM⋅hr in ≥ 80% of patients (day 1 and end of therapy) − Based on Phase 1 data and modeling and simulation, a sample size of 30 patients would be sufficient − Evaluate safety and tolerability in patients receiving dosing regimens predicted to be efficacious Secondary Objective Efficacy − Regimen of micafungin followed by oral SCY-078 in the overall population − SCY-078 in subset of patients with C. glabrata and C. krusei − Will assess results in context of prior invasive candidiasis studies 12
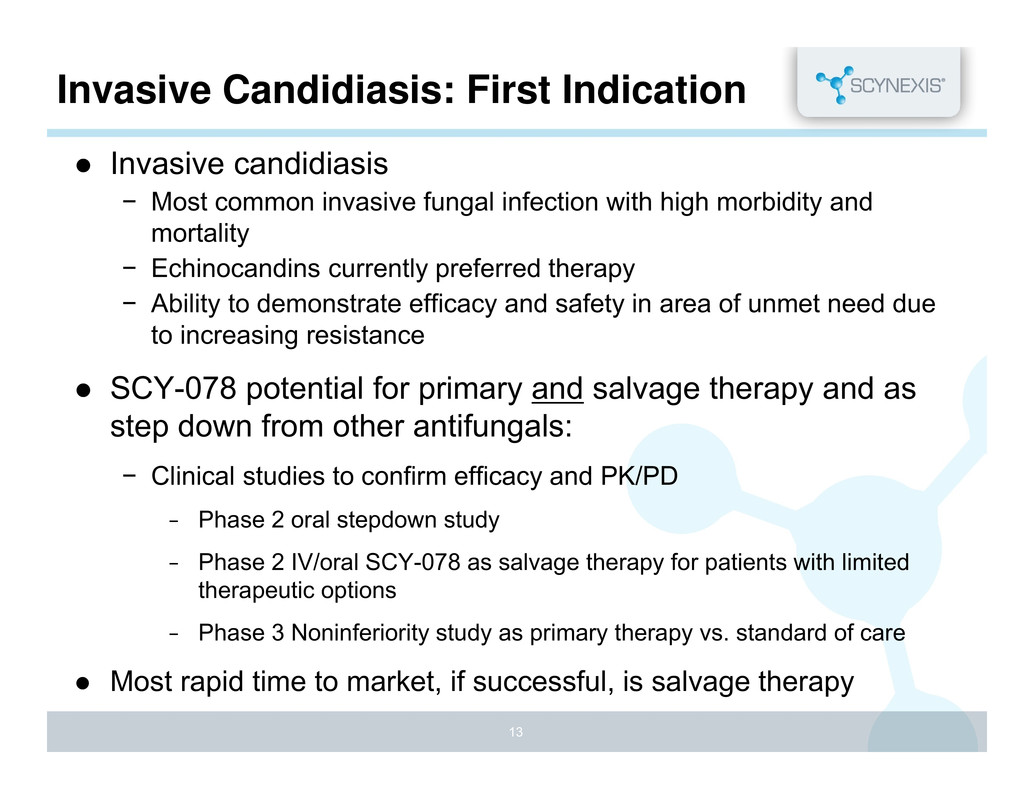
Invasive Candidiasis: First Indication Invasive candidiasis − Most common invasive fungal infection with high morbidity and mortality − Echinocandins currently preferred therapy − Ability to demonstrate efficacy and safety in area of unmet need due to increasing resistance SCY-078 potential for primary and salvage therapy and as step down from other antifungals: − Clinical studies to confirm efficacy and PK/PD − Phase 2 oral stepdown study − Phase 2 IV/oral SCY-078 as salvage therapy for patients with limited therapeutic options − Phase 3 Noninferiority study as primary therapy vs. standard of care Most rapid time to market, if successful, is salvage therapy 13
Additional Indications Invasive Aspergillosis − Salvage therapy in patients refractory to or intolerant of approved treatment (data support indication for prophylaxis, pre-emptive therapy) − Option to explore first line treatment compared to voriconazole Prevention of Candida and Aspergillus infections in high risk patients Pediatrics 14
Projected Milestones SCY-078 for invasive antifungal infections − First patient enrolled in oral formulation Phase 2 study for the treatment of invasive Candida infection 1Q--15 − IV formulation selection and IND-enabling studies 1H-15 − FIM IV formulation 2H-15 − Complete Phase 2 Data 1H-16 Monetization of non-core assets 15

Financials Initial Public Offering May 2, 2014 − $62mm Raised − Top-tier Life Sciences Investors Cash Balance $34mm as of September 30, 2014 − Funds SCY-078 Development Through Q1-2016 − Non-Core Assets Potential Source of Non-Dilutive Capital Share Count − 8.5mm Shares Outstanding − 9.3mm Fully Diluted 16
Management Team CEO and a Member of Board of Directors since 1999 20-year international pharmaceutical career with Aventis Pharma and Rhône-Poulenc Rorer Former Infectious Diseases Director Yves Ribeill, PhD President & CEO CFO since November 2003 Former CFO of Nobex Corporation and VP of Finance for International Murex Technologies Certified Public Accountant Chuck Osborne, Jr. Chief Financial Officer Management team with significant experience in drug discovery and development CMO since January 2014 Former VP Infectious Disease Research and later VP Project Leadership, Neuroscience at Merck Developed Cancidas through approval at Merck Former CMO Novexel SA Carole Sable, MD Chief Medical Officer 17
SCYNEXIS Key Highlights 18 Pharmaceutical company committed to the discovery, development and commercialization of novel anti-infectives to address significant unmet therapeutic needs SCY-078 Ph II, novel, next generation antifungal addressing a large and growing unmet medical need of increasing antifungal resistance Strategic partnering and monetization of non-core assets Strong cash position through Q1-2016 Experienced and proven management team Enfumafungin 1st non-azole with both IV and oral formulation Broad activity against Candida and Aspergillus spp FDA QIDP and Fast Track Designations