
Confidential Draft Submission No. 2 submitted to the Securities and Exchange Commission on January 29, 2014. This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
SCYNEXIS, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 56-2181648 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
3501 C Tricenter Boulevard
Durham, North Carolina 27713
(919) 544-8600
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Yves J. Ribeill, Ph.D.
President and Chief Executive Officer
SCYNEXIS, Inc.
3501 C Tricenter Boulevard
Durham, North Carolina 27713
(919) 544-8600
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Matthew B. Hemington Brett D. White Cooley LLP 3175 Hanover Street Palo Alto, California 94304 (650) 843-5000 |
Eileen C. Pruette General Counsel SCYNEXIS, Inc. 3501 C Tricenter Boulevard Durham, North Carolina 27713 (919) 544-8600 |
Curtis L. Mo Torrie C. Nute DLA Piper LLP (US) 2000 University Avenue East Palo Alto, California 94303 (650) 833-2000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | þ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ |
CALCULATION OF REGISTRATION FEE
|
| ||||
| Title of Each Class of Securities to be Registered |
Proposed Maximum Aggregate Offering Price(1)(2) |
Amount of Registration Fee(3) | ||
| Common Stock, $0.001 par value per share |
||||
|
| ||||
|
| ||||
| (1) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. |
| (2) | Includes offering price of any additional shares that the underwriters have the over-allotment option to purchase. |
| (3) | Calculated pursuant to Rule 457(o) under the Securities Act of 1933, as amended, based on an estimate of the proposed maximum aggregate offering price. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JANUARY 29, 2014
PRELIMINARY PROSPECTUS
Shares

SCYNEXIS, Inc.
Common Stock
We are offering shares of our common stock. This is our initial public offering and no public market currently exists for our common stock. We expect the initial public offering price to be between $ and $ per share. We have applied to list our common stock on the NASDAQ Global Market under the symbol “SCYX.”
Investing in our common stock involves a high degree of risk. Please read “Risk Factors” beginning on page 9 of this prospectus.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, and are subject to reduced public company reporting requirements.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds to SCYNEXIS, Inc., |
$ | $ | ||||||
| (1) | See “Underwriting” beginning on page 150 for a full description of compensation payable to the underwriters. |
Delivery of the shares of common stock is expected to be made on or about , 2014. We have granted the underwriters an option for a period of 30 days to purchase an additional shares of our common stock to cover over-allotments. If the underwriters exercise the option in full, the total underwriting discounts and commissions payable by us will be $ , and the total proceeds to us, before expenses, will be $ .
RBC CAPITAL MARKETS
Prospectus dated , 2014
We and the underwriters have not authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is accurate only as of its date regardless of the time of delivery of this prospectus or of any sale of common stock.
Until and including , 2014 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
Neither we nor the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons who come into possession of this prospectus and any free writing prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus and any free writing prospectus applicable to that jurisdiction.
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before deciding to invest in our common stock, you should read this entire prospectus carefully, including the sections of this prospectus titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes. Unless the context otherwise requires, references in this prospectus to the “company,” “SCYNEXIS,” “we,” “us” and “our” refer to SCYNEXIS, Inc.
Overview
SCYNEXIS is a pharmaceutical company committed to the discovery, development and commercialization of novel anti-infectives to address significant unmet therapeutic needs. We are developing our lead product candidate, SCY-078, as a novel oral and intravenous (IV) drug for the treatment of serious and life-threatening invasive fungal infections in humans. SCY-078 has been shown to be effective in vitro and in vivo in animal studies against a broad spectrum of medically relevant fungal species, including drug-resistant strains, that account for approximately 85% of invasive fungal infections in the United States and Europe. SCY-078 was shown to be sufficiently safe and well-tolerated in multiple Phase 1 studies to support progression to Phase 2 studies. We anticipate beginning a Phase 2 study in the first half of 2014 with an oral formulation of SCY-078 for the treatment of invasive Candida infection, a common and often fatal invasive fungal infection, and anticipate beginning a Phase 2 study with an IV formulation of SCY-078 in 2015.
The worldwide market for prescription anti-fungal therapeutics, where we will target SCY-078, totaled approximately $5.9 billion in 2011 and is forecasted to grow to $6.5 billion in 2016. Incidence rates of confirmed infection by Candida and Aspergillus species indicate that these two pathogens cause over 450,000 invasive fungal infections each year. The rapid progression of the disease and the high mortality rates associated with invasive fungal infections often result in treatments being administered in unconfirmed cases or as a preventative measure, and we estimate that the total cases treated to be approximately three to four times the number of confirmed cases. Also, there is increasing use of drugs that suppress the immune system, such as chemotherapies or drugs for auto-immune disease and transplantation, which has led to an increased rate of invasive fungal infections. Furthermore, the limited number of anti-fungal drug classes, consisting of azoles, echinocandins and polyenes, and their overuse, has led to increased numbers of, and infections with, drug-resistant strains. The resulting pattern of infection, followed by treatment, followed by the development of resistance, followed by more infections is familiar to the medical community, as it has faced these same issues with multi-drug resistant bacterial infections such as methicillin-resistant Staphylococcus aureus, commonly known as MRSA.
SCY-078 represents a new chemical class of drugs designed to block an established target in infectious fungi. We have conducted studies of SCY-078 using animal models that were used in the development of previously approved anti-fungal drugs where these models were proven to be predictive of efficacy in humans. Using these well-established animal models, SCY-078 was shown to be highly active against Candida and Aspergillus. SCY-078 has shown potent in vitro activity against a large collection of medically relevant strains of Candida and Aspergillus, including multi-drug resistant strains that have been isolated from infected patients. Across seven Phase 1 studies, which included over 100 healthy human volunteers, SCY-078 achieved sustained blood concentrations at levels believed to be clinically relevant (those predicted to have a therapeutic effect) and was sufficiently safe and well tolerated to support progression to Phase 2 studies. We are developing both an IV and oral formulation of SCY-078 because patients are
1
typically prescribed IV treatment in hospitals, and then are switched, or “stepped down,” to oral formulations when the patient shows sufficient improvement of symptoms. The availability of SCY-078 in both oral and IV formulations would allow patients to remain within the same drug class and potentially be discharged from the hospital sooner.
The increasing rates of bacterial and fungal infections and resistance to current therapies, along with associated high rates of mortality, led to the 2012 passage of the Generating Antibiotic Incentives Now (GAIN) Act in the United States. The GAIN Act established incentives for the development of new therapies for serious and life-threatening infections by making streamlined priority review and fast track processes available for drugs which the U.S. Food and Drug Administration, or FDA, designates as Qualified Infectious Disease Products, or QIDPs. The FDA has granted the oral form of SCY-078 QIDP status, which will provide for an additional five years of data exclusivity, providing an additional layer of protection from generic drug competition. We are submitting an additional application for the IV form of SCY-078 which we anticipate will be granted within the 60 day review period. In addition to data exclusivity, SCY-078 is covered by a composition of matter patent extending to 2030. We have exclusive worldwide rights to SCY-078 in the field of human health, and have licensed the rights in Russia and certain smaller non-core markets to R-Pharm, CJSC, or R-Pharm, a leading supplier of hospital drugs in Russia.
As the next step in the development of SCY-078, we plan to conduct a randomized Phase 2 study, scheduled to commence in the first half of 2014. This will be a three arm study comparing two doses of SCY-078 to current standard of care in patients with invasive Candida infections, including patients infected with Candida species which are resistant to azoles, patients previously treated with azole therapy, and treatment-naïve patients. We also intend to initiate a Phase 2 study with an IV formulation of SCY-078 in the first half of 2015 in patients with invasive Candida infections. We anticipate this study will include the option of stepping patients down from IV to oral SCY-078.
If approved, we intend to market SCY-078 to hospitals and major medical centers, where physicians specializing in critical care, infectious disease specialists, and physicians treating immune-compromised or immune-suppressed patients, such as oncologists and those performing solid organ transplants and stem cell transplants are likely to be found and where invasive fungal infections are more prevalent.
Despite the increasing availability of generic azole drugs and the eventual availability of generic echinocandin drugs, we believe SCY-078, once commercialized, will be able to achieve premium branded pricing comparable to that of the top selling branded hospital-based antibiotics. We believe we can achieve attractive premium pricing even with the increasing availability of generic drugs based on the following:
| Ÿ | Drug resistant strains. There are many invasive fungal strains resistant to azole drugs. High rates of morbidity and mortality, and extended hospital stays associated with infections from these resistant strains, will make a strong argument for use of a premium-priced anti-fungal drug which is effective against these resistant strains. |
| Ÿ | Alternative to echinocandins. Physicians are reluctant to prescribe azoles in hospitals where azole resistance is prevalent, as an ineffective course of therapy can compromise the patient’s survival. Thus, in these settings, physicians often prescribe echinocandins; but echinocandins are only available in IV formulation. Subsequent step down to an oral azole to allow release from the hospital risks emergence of an azole resistant infection, leading some physicians to keep patients on IV echinocandins for the full course of therapy. If successfully developed, SCY-078 would provide an attractive alternative to echinocandin therapy by offering an IV-to-oral step-down within a single |
2
| therapeutic class, thereby facilitating earlier discharge from the hospital and the resultant reduced exposure to the risk of hospital-acquired infections. |
In addition to SCY-078, we have clinical and preclinical programs based on the use of cyclophilin inhibitors to treat viral diseases. We also provide contract research and development services primarily in the field of animal health, which currently generate substantially all of our revenue. As a spinout from Aventis in 2000, we began as a chemistry and animal health services company, providing contract research services to third parties. Through the provision of these services, we built significant expertise in parasitic infections and drug discovery. In addition, while we have not previously developed our own compound, we have recently hired a Chief Medical Officer, Carole Sable, M.D., who has substantial experience in the field of anti-infective drug development, to assist us in taking SCY-078 through the clinic. We also have 38 scientists who have Ph.D. degrees and extensive pharmaceutical experience, including our CEO who prior to founding Scynexis was involved in the discovery and development efforts that resulted in the approval of the anti-bacterial Synercid. We intend to leverage this expertise in the development of SCY-078.
Our Corporate Strategy
Key elements of our strategy include:
| Ÿ | further develop SCY-078 to obtain regulatory approval in major commercial markets; |
| Ÿ | commercialize SCY-078 in the United States through a focused hospital-based sales force; |
| Ÿ | contract with commercial partners to develop and commercialize SCY-078 outside of the United States; and |
| Ÿ | leverage our strong scientific team and extensive in-house expertise in human and animal drug development to pursue the development of additional proprietary compounds. |
Risk Factors Associated with Our Business
Our business is subject to numerous risks, as more fully described in the section entitled “Risk Factors” immediately following this prospectus summary. You should read these risks before you invest in our common stock. In particular, our risks include, but are not limited to, the following:
| Ÿ | historically, we have been a preclinical research services company devoting substantially all of our resources and efforts to providing research services to other companies, and we have only recently shifted our focus to developing our own drug candidates, primarily SCY-078; |
| Ÿ | we have never fully developed our own product candidates and we have no products approved for commercial sale; |
| Ÿ | we have never been profitable, and to date we have not generated any revenue from product sales. As a result, our ability to curtail our losses and reach profitability is unproven, and we may never achieve or sustain profitability; |
| Ÿ | we expect a number of factors to cause our operating results to fluctuate on a quarterly and annual basis, which may make it difficult to predict our future performance; |
| Ÿ | we may continue to require substantial additional capital, and if we are unable to raise capital when needed we would be forced to delay, reduce or eliminate our product development programs; |
| Ÿ | we cannot be certain that SCY-078 or any of our other product candidates will receive regulatory approval, and without regulatory approval will not be able to market our product candidates; |
3
| Ÿ | we have limited experience in conducting Phase 2 and Phase 3 clinical trials and have never submitted a new drug application, or NDA, before, and we may be unable to do so for SCY-078 or any future product candidate we may seek to develop; |
| Ÿ | a significant use of anti-fungal drugs is treatment due to the presence of symptoms before diagnosis of the invasive fungal infections, and if a diagnostic tool is developed for the quick diagnosis of invasive fungal infections, the number of treatments using anti-fungal drugs may decrease significantly, decreasing the potential market for SCY-078; and |
| Ÿ | we are substantially dependent on our agreement with Merial for generation of our revenue, and that agreement expires on December 31, 2014. |
Corporate information
We were originally incorporated in Delaware in November 1999 as ScyRex, Inc. We subsequently changed our name to SCYNEXIS Chemistry & Automation, Inc. in April 2000 and to SCYNEXIS, Inc. in June 2002. Our principal executive offices are located at 3501 C Tricenter Boulevard, Durham, North Carolina 27713, and our telephone number is (919) 544-8600. Our website address is www.scynexis.com. The information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our common stock.
“SCYNEXIS,” our logo and other trade names, trademarks and service marks of SCYNEXIS appearing in this prospectus are the property of SCYNEXIS. Other trade names, trademarks, and service marks appearing in this prospectus are the property of their respective holders.
Implications of Being an Emerging Growth Company
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or JOBS Act. As such, we are eligible for exemptions from various reporting requirements applicable to other public companies that are not emerging growth companies, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 and reduced disclosure obligations regarding executive compensation. The JOBS Act also provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. We intend to avail ourselves of all other exemptions.
We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of this offering, (b) in which we have total annual gross revenue of at least $1.0 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period.
4
The Offering
| Common stock offered by us |
shares |
| Common stock to be outstanding immediately after this offering |
shares |
| Underwriters’ over-allotment option |
The underwriters have an option to purchase up to additional shares of common stock to cover over-allotments as described in “Underwriting.” |
| Use of proceeds |
We estimate that the net proceeds from the issuance of our common stock in this offering will be approximately $ million, or approximately $ million if the underwriters exercise their over-allotment option in full, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
| We intend to use approximately $ million for clinical and preclinical costs associated with the completion of Phase 2 trials and the initiation of Phase 3 trials for our lead product candidate SCY-078, approximately $7.5 million to pay down a portion of our $15.0 million credit facility upon the closing of this offering, with the balance to be paid down as it becomes due, and the remainder for working capital, capital expenditures and other general corporate purposes. See “Use of Proceeds” for additional information. |
| Risk factors |
See “Risk Factors” beginning on page 9 and the other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our common stock. |
| Proposed NASDAQ Global Market symbol |
We intend to apply for listing of our common stock on the NASDAQ Global Market under the symbol “SCYX.” |
The number of shares of our common stock to be outstanding after this offering is based on 40,720,182 shares of our common stock outstanding as of December 11, 2013 (including convertible preferred stock on an as-converted basis), and excludes the following:
| Ÿ | 2,649,528 shares of our common stock issuable upon the exercise of stock options outstanding at a weighted-average exercise price of $1.16 per share; |
| Ÿ | 1,172,284 shares of our common stock reserved for future issuance under our 2009 Stock Option Plan; |
| Ÿ | shares of our common stock reserved for future issuance under our 2014 Equity Incentive Plan; |
| Ÿ | shares of our common stock reserved for future issuance under our 2014 Employee Stock Purchase Plan; and |
5
| Ÿ | 5,531,344 shares of our common stock issuable upon the exercise of common stock warrants and convertible preferred stock warrants outstanding at a weighted-average exercise price of $0.13 per share. |
Unless otherwise indicated, all information in this prospectus reflects and assumes the following:
| Ÿ | a for reverse split of our common stock to be effected prior to the effectiveness of this offering; |
| Ÿ | the automatic conversion of all outstanding shares of our convertible preferred stock into an aggregate of 33,904,001 shares of our common stock immediately prior to the closing of this offering; |
| Ÿ | the automatic conversion of all outstanding convertible preferred stock warrants into warrants to purchase an aggregate of 283,147 shares of our common stock immediately prior to the closing of this offering; |
| Ÿ | the filing of our amended and restated certificate of incorporation and the adoption of our amended and restated bylaws immediately prior to the closing of this offering; and |
| Ÿ | no exercise of the underwriters’ over-allotment option to purchase up to additional shares of our common stock. |
6
Summary Financial Data
The following tables summarize our financial data and should be read together with the sections in this prospectus titled “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
We have derived the statement of operations data for the years ended December 31, 2012 and 2011, from our audited financial statements included elsewhere in this prospectus. We have derived the statement of operations data for the nine months ended September 30, 2013 and 2012, and the balance sheet data as of September 30, 2013, from our unaudited financial statements included elsewhere in this prospectus. We have prepared the unaudited financial statements on the same basis as the audited financial statements and have included, in our opinion, all adjustments, consisting only of normal recurring adjustments, that we consider necessary for a fair presentation of the financial information set forth in those financial statements. Our historical results are not necessarily indicative of the results that should be expected in the future, and our interim results are not necessarily indicative of the results that should be expected for the full year or any other period.
| Nine Months ended September 30, |
Year ended December 31, |
|||||||||||||||
| 2013 | 2012 | 2012 | 2011 | |||||||||||||
| (in thousands, except share and per share data) | ||||||||||||||||
| Statement of operations data: |
||||||||||||||||
| Total revenue |
$ | 13,184 | $ | 11,988 | $ | 16,837 | $ | 26,454 | ||||||||
| Cost of revenue |
12,531 | 10,690 | 14,364 | 17,753 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
653 | 1,298 | 2,473 | 8,701 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
3,203 | 6,977 | 8,927 | 11,633 | ||||||||||||
| Selling, general and administrative |
3,150 | 3,742 | 4,742 | 4,980 | ||||||||||||
| Gain on sale of asset |
(988 | ) | (3,412 | ) | (3,412 | ) | — | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
5,365 | 7,307 | 10,257 | 16,613 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(4,712 | ) | (6,009 | ) | (7,784 | ) | (7,912 | ) | ||||||||
| Other (expense) income: |
||||||||||||||||
| Amortization of deferred financing cost and debt discount |
(2,504 | ) | (2,141 | ) | (2,918 | ) | (2,138 | ) | ||||||||
| Interest expense-related party |
(703 | ) | (516 | ) | (747 | ) | (29 | ) | ||||||||
| Interest expense |
(142 | ) | (172 | ) | (225 | ) | (170 | ) | ||||||||
| Derivative fair value adjustment |
(671 | ) | 330 | 185 | 20 | |||||||||||
| Other (expense) income |
— | (8 | ) | 12 | 23 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other expense |
(4,020 | ) | (2,507 | ) | (3,693 | ) | (2,294 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (8,732 | ) | $ | (8,516 | ) | $ | (11,477 | ) | $ | (10,206 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share: |
||||||||||||||||
| Basic and diluted |
$ | (1.27 | ) | $ | (1.30 | ) | $ | (1.73 | ) | $ | (2.53 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted, pro forma(1) |
$ | $ | ||||||||||||||
|
|
|
|
|
|||||||||||||
| Weighted average common shares outstanding: |
||||||||||||||||
| Basic and diluted |
6,852,981 | 6,573,329 | 6,642,837 | 4,034,720 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted, pro forma(1) |
||||||||||||||||
|
|
|
|
|
|||||||||||||
| Stock-based compensation expense included above: |
||||||||||||||||
| Cost of revenue |
$ | 27 | $ | 26 | $ | 103 | $ | 116 | ||||||||
| Research and development |
16 | 10 | 40 | 47 | ||||||||||||
| Selling, general and administrative |
67 | 123 | 215 | 219 | ||||||||||||
7
| (1) | Pro forma basic and diluted net loss per share have been calculated assuming the conversion of all outstanding shares of convertible preferred stock, the conversion of all outstanding convertible promissory notes and the exercise of all warrants issued with our convertible notes into an aggregate of shares of common stock as of the beginning of the applicable period or at the time of issuance, if later. |
| As of September 30, 2013 | ||||||||||||
| Actual | Pro forma(1) |
Pro forma as adjusted(2)(3) |
||||||||||
| (in thousands) | ||||||||||||
| Balance sheet data: |
||||||||||||
| Cash and cash equivalents |
$ | 926 | $ | $ | ||||||||
| Working capital (deficit) |
(13,402 | ) | ||||||||||
| Total assets |
12,146 | |||||||||||
| Total stockholders’ deficit |
(70,105 | ) | ||||||||||
| (1) | The pro forma column reflects the conversion of all outstanding shares of our convertible preferred stock into an aggregate of shares of common stock immediately prior to the closing of this offering. In addition, it reflects the exercise of all common stock warrants issued with our convertible notes into an aggregate of shares of common stock immediately prior to the closing of this offering and the resulting reclassification of a derivative liability of $ related to those common stock warrants to reduce stockholders’ deficit. |
| (2) | The pro forma as adjusted column reflects the pro forma adjustments described in footnote (1) above and the sale by us of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
| (3) | A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) each of pro forma as adjusted cash and cash equivalents, working capital and total assets by $ and decrease (increase) pro forma as adjusted total stockholders’ deficit by $ , assuming the number of shares we are offering, as set forth on the cover page of this prospectus, remains the same, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase (decrease) of in the number of shares we are offering would increase (decrease) each of pro forma as adjusted cash and cash equivalents, working capital and total assets by approximately $ and decrease pro forma as adjusted stockholders’ deficit by approximately $ , assuming the assumed initial public offering price per share remains the same. The pro forma as adjusted information is illustrative only, and we will adjust this information based on the actual initial public offering price, number of shares offered and other terms of this offering determined at pricing. |
8
Investing in our common stock involves a high degree of risk. Before you invest in our common stock, you should carefully consider the following risks, as well as general economic and business risks and all of the other information contained in this prospectus. Any of the following risks could have a material adverse effect on our business, operating results and financial condition and cause the trading price of our common stock to decline, which would cause you to lose all or part of your investment. When determining whether to invest, you should also refer to the other information contained in this prospectus, including our financial statements and the related notes thereto.
Risks Relating to Our Financial Condition and Need for Additional Capital
We have never been profitable, we have no products approved for commercial sale, and to date we have not generated any revenue from product sales. As a result, our ability to curtail our losses and reach profitability is unproven, and we may never achieve or sustain profitability.
We are not profitable and do not expect to be profitable in the foreseeable future. We have incurred net losses in each year since our inception, including net losses of approximately $8.7 million, $11.5 million and $10.2 million for the nine months ended September 30, 2013, and for the years ended December 31, 2012 and 2011, respectively. As of September 30, 2013, we had an accumulated deficit of approximately $91.5 million. Although we have generated revenues through our contract research and development services, these revenues have not been sufficient to support our business, and so in addition we have financed our operations through the sale of convertible preferred stock and convertible debt. We intend to devote a majority of our financial resources to the development of SCY-078, our lead product candidate, and to a much lesser extent to development of product candidates from our cyclophilin inhibitor platform. We have not generated any revenue from product sales. The report of our independent registered public accounting firm on our financial statements for the year ended December 31, 2012, includes an explanatory paragraph relating to our ability to continue as a going concern. We have suffered substantial losses from operations and require additional financing. Ultimately we need to generate additional revenues and attain profitable operations. These factors raise substantial doubt about our ability to continue as a going concern.
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. The net losses we incur may fluctuate significantly from quarter to quarter. We anticipate that our expenses will increase substantially as we:
| Ÿ | continue the development of SCY-078; |
| Ÿ | initiate clinical trials for SCY-078; |
| Ÿ | seek marketing approvals for SCY-078; |
| Ÿ | establish a sales, marketing and distribution infrastructure to commercialize any products for which we may obtain marketing approval; |
| Ÿ | maintain, expand and protect our intellectual property portfolio; |
| Ÿ | hire additional clinical, quality control and scientific personnel; and |
| Ÿ | create additional infrastructure to support our operations as a public company. |
In addition, our expenses could increase if we are required by the U.S. Food and Drug Administration, or the FDA, to perform studies in addition to, or that are larger than, those that we currently expect.
As a result of the foregoing, we expect to experience net losses and negative cash flows for the foreseeable future, and we are unable to predict when, or if, we will be able to achieve profitability. Our
9
losses and negative cash flows have had, and will continue to have, an adverse effect on our stockholders’ equity, financial position and working capital.
We expect a number of factors to cause our operating results to fluctuate on a quarterly and annual basis, which may make it difficult to predict our future performance.
Our financial condition and operating results have varied significantly in the past and will continue to fluctuate from quarter to quarter or year to year due to a variety of factors, many of which are beyond our control. The following factors relating to our business, as well as factors described elsewhere in this prospectus, may contribute to these fluctuations:
| Ÿ | the costs associated with developing SCY-078, which are difficult for us to predict; |
| Ÿ | any delays in regulatory review and approval of SCY-078; |
| Ÿ | delays in the timing of filing of a new drug application, or NDA, as well as commencement, enrollment and the timing of clinical testing, of SCY-078 or any other product candidates we may seek to develop; |
| Ÿ | our ability to commercialize product candidates, both in the United States and overseas, if we are able to obtain regulatory approval to do so; |
| Ÿ | the costs associated with obtaining and maintaining regulatory approval and ongoing company compliance and product compliance for SCY-078; |
| Ÿ | the success of our providing contract research and development services; |
| Ÿ | market acceptance of SCY-078 and any future product candidates we may seek to develop; |
| Ÿ | changes in regulations and regulatory policies; |
| Ÿ | competition from existing products or new products that may emerge; |
| Ÿ | the ability of patients or healthcare providers to obtain coverage of, or sufficient reimbursement for, any products we are able to develop; |
| Ÿ | our ability to establish or maintain collaborations, licensing or other arrangements; |
| Ÿ | costs related to, and outcomes of, potential litigation; |
| Ÿ | potential product liability claims; and |
| Ÿ | potential liabilities associated with hazardous materials. |
Due to the various factors mentioned above, and others, the results of any quarterly or annual periods should not be relied upon as indications of future operating performance.
We may continue to require substantial additional capital, and if we are unable to raise capital when needed we would be forced to delay, reduce or eliminate our development program for SCY-078.
Developing pharmaceutical products, including conducting preclinical studies and clinical trials, is expensive. If the FDA requires that we perform additional studies beyond those that we currently expect, our expenses could increase beyond what we currently anticipate and the timing of any potential product approval may be delayed. We estimate that the net proceeds from this offering will be approximately $ million, assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated expenses payable by us. We believe that the net proceeds from this offering will be sufficient to
10
meet our anticipated operating requirements through . However, changing circumstances may cause us to consume capital more rapidly than we currently anticipate. We may need to raise additional funds from the issuance of equity and/or debt securities or otherwise obtain funding through strategic alliances or collaborations with third parties. In any event, we will require additional capital to complete development of, to seek regulatory approval for and, if approval is obtained, to commercialize SCY-078 and any future product candidates we may seek to develop. Raising funds in the current economic environment, when the capital markets have been affected by the global recession, may present additional challenges.
If we are required to secure additional financing, the additional fundraising efforts may divert our management from our day-to-day activities, which may adversely affect our ability to develop and commercialize SCY-078 and any future product candidates we may seek to develop. In addition, we cannot guarantee that financing will be available in sufficient amounts or on terms acceptable to us, if at all. If we are unable to raise additional capital when required or on acceptable terms, we may be required to:
| Ÿ | significantly delay, scale back or discontinue the development or commercialization of SCY-078 and any future product candidates we may seek to develop; |
| Ÿ | seek strategic alliances for research and development programs at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available; or |
| Ÿ | relinquish or license on unfavorable terms our rights to any product candidates that we otherwise would seek to develop or commercialize ourselves. |
If we are required to conduct additional fundraising activities and we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we will be prevented from pursuing development and commercialization efforts, which will have a material adverse effect on our business, operating results and prospects.
Risks Relating to the Development, Regulatory Approval and Commercialization
of Our Product Candidates For Human Use
Historically we have been primarily a contract research and development services company devoting a majority of our resources and efforts to providing research and development services to other companies, and we are only now shifting our focus to developing our own drug candidate SCY-078.
We were spun out from Aventis S.A., or Aventis, in 2000 as a chemistry and animal health services company, providing contract research services to third parties. Since then, we have derived substantially all of our revenue from providing these services to human and animal health companies to assist them in developing their own drug candidates. In the course of providing these services, we have leveraged the expertise to develop our own proprietary compounds, including a platform of cyclophilin inhibitors, among them SCY-635. In 2013, under the contract with Merck Sharp & Dohme Corp., or Merck, a subsidiary of Merck & Co., Inc., Merck exclusively licensed SCY-078 to us in the field of human health and in conjunction with that license transferred to us the investigational new drug application pending with the FDA and related regulatory responsibilities, as well as all data Merck had developed for the compound, plus active pharmaceutical ingredients and tablets. In 2014, Merck assigned the patents to us related to SCY-078 that it had exclusively licensed to us.
Although we have conducted Phase 1 and Phase 2 studies of SCY-635, our cyclophilin inhibitor, we only acquired the rights to develop SCY-078, our lead drug candidate for the treatment of invasive fungal infections, in May 2013. We do not have a significant history of developing our own drug candidates, and we have not brought any drug candidates to market, which makes it difficult to assess our ability to develop and commercialize SCY-078 and any future product candidates we may seek to develop or commercialize.
11
We cannot be certain that SCY-078 will receive regulatory approval, and without regulatory approval we will not be able to market SCY-078. Regulatory approval is a lengthy, expensive and uncertain process.
Our ability to generate significant revenue related to SCY-078 sales will depend on the successful development and regulatory approval of SCY-078. We expect that the earliest that we could obtain regulatory approval of SCY-078 and commence commercialization of SCY-078 will be several years from now, if at all.
We currently have no products approved for sale and we cannot guarantee that we will ever have marketable products. The development and commercialization of a product candidate, including preclinical and clinical testing, manufacturing, quality systems, labeling, approval, record-keeping, selling, promotion, marketing and distribution of products, is subject to extensive regulation by the FDA in the United States and regulatory authorities in other countries, with regulations differing from country to country. We are not permitted to market product candidates in the United States until and unless we receive approval of an NDA from the FDA. We have not submitted an NDA for SCY-078. Obtaining approval of an NDA is a lengthy, expensive and uncertain process. An NDA must include extensive preclinical and clinical data and supporting information to establish the product candidate’s safety and effectiveness for each indication. The approval application must also include significant information regarding the chemistry, manufacturing and controls for the product. The regulatory development and review process typically takes years to complete, involves numerous uncertainties and the potential for concerns to emerge late in the development process, and approval is never guaranteed. Even if a product is approved, the FDA may limit the indications for which the product may be used, include extensive warnings on the product labeling or require costly ongoing requirements for post-marketing clinical studies and surveillance or other risk management measures to monitor the safety or efficacy of the product candidate. Markets outside of the United States also have requirements for approval of drug candidates with which we must comply prior to marketing. Obtaining regulatory approval for marketing of a product candidate in one country does not ensure we will be able to obtain regulatory approval in other countries, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in other countries. Also, any regulatory approval of a product candidate, once obtained, may be withdrawn. If SCY-078 or any of our other wholly-owned or partnered product candidates do not receive regulatory approval, we may not be able to generate sufficient revenue to become profitable or to continue our operations. Moreover, the filing of our NDA or the receipt of regulatory approval does not assure commercial success of any approved product.
Although the oral form of SCY-078 has been granted Qualified Infectious Disease Product status, this does not guarantee that the length of the FDA review process will be significantly shorter than otherwise, or that SCY-078 will ultimately be approved by the FDA.
We applied to the FDA for, and received, the designation of the oral form of SCY-078 as a Qualified Infectious Disease Product, or QIDP, under the Generating Antibiotic Incentive Now Act, or GAIN Act. We are submitting an additional application for the IV form of SCY-078 which we anticipate will be granted within the 60 day review period. There is no guarantee that the IV form of SCY-078 will be granted. We anticipate that the QIDP designation will provide, among other benefits, an overall increased level of communication with the FDA during the development process as a fast track product, priority review once a NDA is submitted, and, if SCY-078 is approved for its proposed use and awarded five years of exclusivity as a new chemical entity, or NCE, SCY-078 will be eligible for a ten year period of data exclusivity, comprising five years of NCE exclusivity plus an additional five years as a designated QIDP. This exclusivity period should protect SCY-078 from being referenced in an abbreviated new drug application, or ANDA, in support of a generic drug, or a 505(b)(2) new drug application for a follow-on product until the expiration of the
12
exclusivity period (which may be shortened by one year if an ANDA or 505(b)(2) applicant seeks to challenge any of the patents that claim SCY-078). However, the primary framework of the GAIN Act became effective July 9, 2012, and as a relatively new law there is limited precedent for the way in which it will be implemented. Receipt of QIDP designation in practice may not result in a faster development process, review or approval compared to drugs considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA or related exclusivity benefits.
Delays in the commencement, enrollment and completion of clinical trials could result in increased costs to us and delay or limit our ability to obtain regulatory approval for SCY-078 or any future product candidates.
We do not know whether clinical trials of SCY-078 or any future product candidates we may seek to develop will be allowed to commence or, if commenced, will be completed on schedule or at all. The commencement, enrollment and completion of clinical trials can be delayed for a variety of reasons, including:
| Ÿ | inability to reach agreements on acceptable terms with prospective clinical research organizations, or CROs, and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| Ÿ | difficulty identifying and engaging qualified clinical investigators; |
| Ÿ | regulatory objections to commencing a clinical trial or proceeding to the next phase of investigation, including inability to reach agreement with the FDA or non-U.S. regulators regarding the scope or design of our clinical trials or for other reasons such as safety concerns that might be identified during preclinical development or early stage clinical trials; |
| Ÿ | inability to identify and maintain a sufficient number of trial sites, many of which may already be engaged in other clinical trial programs, including some that may be for the same indication as our product candidates; |
| Ÿ | withdrawal of clinical trial sites from our clinical trials as a result of changing standards of care or the ineligibility of a site to participate in our clinical trials; |
| Ÿ | inability to obtain institutional review board approval to conduct a clinical trial at prospective sites; |
| Ÿ | difficulty recruiting and enrolling patients to participate in clinical trials for a variety of reasons, including meeting the enrollment criteria for our study and competition from other clinical trial programs for the same indication as product candidates we seek to commercialize; |
| Ÿ | inability to retain patients in clinical trials due to the treatment protocol, personal issues, side effects from the therapy or lack of efficacy, particularly for those patients receiving a placebo; and |
| Ÿ | inability to obtain sufficient funding to commence a clinical trial. |
In addition, a clinical trial may be suspended or terminated by us, our current or any future partners, the FDA or other regulatory authorities due to a number of factors, including:
| Ÿ | failure by us, CROs or clinical investigators to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
| Ÿ | failed inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities; |
| Ÿ | unforeseen safety or efficacy issues or any determination that a clinical trial presents unacceptable health risks; or |
13
| Ÿ | lack of adequate funding to continue the clinical trial due to unforeseen costs resulting from enrollment delays, requirements to conduct additional trials and studies, increased expenses associated with the services of our CROs and other third parties, or other reasons. |
If we are required to conduct additional clinical trials or other testing of SCY-078 or any future product candidates we may seek to develop, we may be delayed in obtaining, or may not be able to obtain, marketing approval for these product candidates.
In addition, if our current or any future partners have rights to and responsibility for development of SCY-078 or any future product candidates, they may fail to meet their obligations to develop and commercialize the product candidates, including clinical trials for these product candidates.
Changes in regulatory requirements and guidance may occur and we or any of our partners may be required by appropriate regulatory authorities to amend clinical trial protocols to reflect these changes. Amendments may require us or any of our partners to resubmit clinical trial protocols to independent review boards for re-examination, which may impact the costs, timing or successful completion of a clinical trial. If we or any of our partners experience delays in the completion of, or if we or our partners terminate, clinical trials, the commercial prospects for SCY-078 and any future product candidates we may seek to develop will be harmed, and our ability to generate revenue from sales of these product candidates will be prevented or delayed. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of a product candidate.
Clinical failure can occur at any stage of clinical development. Because the results of earlier clinical trials are not necessarily predictive of future results, any product candidate we or our current or potential future partners advance through clinical trials may not have favorable results in later clinical trials or receive regulatory approval.
Clinical failure can occur at any stage of our clinical development. Clinical trials may produce negative or inconclusive results, and we or our partners may decide, or regulators may require us, to conduct additional clinical or preclinical testing. In addition, data obtained from tests are susceptible to varying interpretations, and regulators may not interpret data as favorably as we do, which may delay, limit or prevent regulatory approval. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidate. Frequently, product candidates that have shown promising results in early clinical trials have subsequently suffered significant setbacks in later clinical trials. In addition, the design of a clinical trial can determine whether its results will support approval of a product application, or approval of a supplemental application to add a new indication or other changes and flaws or shortcomings in the design of a clinical trial may not become apparent until the clinical trial is well advanced. We have limited experience in designing clinical trials and may be unable to design and execute a clinical trial to support regulatory approval, or approval of supplemental applications for new indications or other changes. Further, clinical trials of potential products often reveal that it is not practical or feasible to continue development efforts. If SCY-078 or any future product candidates are found to be unsafe or lack efficacy, we or our collaborators will not be able to obtain regulatory approval for them and our business would be harmed. For example, if the results of our planned Phase 2 and Phase 3 clinical trials of SCY-078 do not achieve, to the satisfaction of regulators, the primary efficacy endpoints and demonstrate an acceptable level of safety, the prospects for approval of SCY-078 would be materially and adversely affected. A number of companies in the pharmaceutical industry, including those with greater resources and experience than us, have suffered significant setbacks in Phase 2 and Phase 3 clinical trials, even after seeing promising results in earlier clinical trials.
14
In some instances, there can be significant variability in safety and/or efficacy results between different trials of the same product candidate due to numerous factors, including differences in trial protocols and design, differences in size and type of the patient populations, adherence to the dosing regimen and the rate of dropout among clinical trial participants. Further, the patients taking SCY-078 often have other significant medical issues, such as organ transplants, cancer or other conditions in which their immune systems are depressed, which makes it difficult to measure the effect of SCY-078 in the presence of these medical issues. We do not know whether any Phase 2, Phase 3 or other clinical trials we or any partners may conduct will demonstrate consistent and/or adequate efficacy and safety to obtain regulatory approval to market SCY-078 and any future product candidates we may seek to develop.
We have limited experience in conducting Phase 2 and Phase 3 clinical trials and have never submitted an NDA before, and we may be unable to do so for SCY-078 or any future product candidate we may seek to develop.
Merck completed seven Phase 1 clinical trials of SCY-078, and we are planning to conduct Phase 2 and Phase 3 clinical trials of SCY-078. The conduct of successful Phase 2 and Phase 3 clinical trials is essential in obtaining regulatory approval, and the submission of a successful NDA is a complicated process. We have limited experience in preparing and submitting regulatory filings, have previously only sponsored one Phase 2 clinical trial, and have not previously sponsored any Phase 3 clinical trials nor have we ever submitted an NDA before. Consequently, we may be unable to successfully and efficiently execute and complete these planned clinical trials in a way that is acceptable to the FDA and leads to an NDA submission, acceptance and approval of SCY-078 or any future product candidate we may seek to develop. We may require more time and incur greater costs than our competitors and may not succeed in obtaining regulatory approvals of product candidates that we may seek to develop. In addition, failure to commence or complete, or delays in, our planned clinical trials would prevent us from or delay us in commercializing SCY-078 or any future product candidate we may develop.
We have not yet finalized the protocol for our planned Phase 2 study or studies of SCY-078, and are still in discussions with the FDA regarding anticipated indications and study endpoints.
Following the transfer by Merck to us of ownership and responsibility for the clinical development and NDA related to SCY-078, we assessed the regulatory history and initiated discussions with the FDA to obtain clarity on several open questions regarding the clinical development plan for SCY-078. Our most recent meeting with the FDA was in September 2013, and while we obtained feedback at this meeting, there are still some open questions under consideration by the FDA and our Phase 2 protocol is still being finalized. We do not know when, if at all, we will be able to finalize the protocol.
The environment in which our regulatory submissions may be reviewed changes over time, which may make it more difficult to obtain regulatory approval of any of our product candidates we may seek to develop or commercialize.
The environment in which our regulatory submissions are reviewed changes over time. For example, average review times at the FDA for NDAs have fluctuated over the last ten years, and we cannot predict the review time for any submission with any regulatory authorities. Review times can be affected by a variety of factors, including budget and funding levels and statutory, regulatory and policy changes. Moreover, in light of widely publicized events concerning the safety risk of certain drug products, regulatory authorities, members of Congress, the Government Accountability Office, medical professionals and the general public have raised concerns about potential drug safety issues. These events have resulted in the withdrawal of drug products, revisions to drug labeling that further limit use of the drug products and establishment of risk evaluation and mitigation strategies that may, for instance, restrict distribution of drug
15
products. The increased attention to drug safety issues may result in a more cautious approach by the FDA to clinical trials. Data from preclinical studies and clinical trials may receive greater scrutiny with respect to safety, which may make the FDA or other regulatory authorities more likely to terminate clinical trials before completion, or require longer or additional clinical trials that may result in substantial additional expense, a delay or failure in obtaining approval or approval for a more limited indication than originally sought.
In addition, data obtained from preclinical studies and clinical trials are subject to different interpretations, which could delay, limit or prevent regulatory review or approval of product candidates. Changes in FDA personnel responsible for review of our submissions could also impact the manner in which our data are viewed. Furthermore, regulatory attitudes towards the data and results required to demonstrate safety and efficacy can change over time and can be affected by many factors, such as the emergence of new information, including on other products, policy changes and agency funding, staffing and leadership. We do not know whether future changes to the regulatory environment will be favorable or unfavorable to our business prospects.
If SCY-078 or any other future product candidates for which we receive regulatory approval do not achieve broad market acceptance, the revenue that is generated from their sales will be limited.
The commercial success of SCY-078 or any other product candidates we may seek to develop will depend upon the acceptance of these products candidates among physicians, patients, the medical community and healthcare payors. The degree of market acceptance of product candidates will depend on a number of factors, including:
| Ÿ | limitations or warnings contained in the FDA-approved labeling; |
| Ÿ | changes in the standard of care for the targeted indications; |
| Ÿ | limitations in the approved indications; |
| Ÿ | availability of alternative therapies with potentially advantageous results, or other products with similar results at similar or lower cost, including generics and over-the-counter products; |
| Ÿ | lower demonstrated clinical safety or efficacy compared to other products; |
| Ÿ | occurrence of significant adverse side effects; |
| Ÿ | ineffective sales, marketing and distribution support; |
| Ÿ | lack of availability of reimbursement from managed care plans and other third-party payors; |
| Ÿ | timing of market introduction and perceived effectiveness of competitive products; |
| Ÿ | lack of cost-effectiveness; |
| Ÿ | adverse publicity about our product candidates or favorable publicity about competitive products; |
| Ÿ | lack of convenience and ease of administration; and |
| Ÿ | potential product liability claims. |
If SCY-078 or any future product candidates we may seek to develop are approved, but do not achieve an adequate level of acceptance by physicians, healthcare payors and patients, sufficient revenue may not be generated from these product candidates, and we may not become or remain profitable. In addition, efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may never be successful.
16
A significant use of anti-fungal drugs is treatment due to the presence of symptoms before diagnosis of the invasive fungal infections, and if a diagnostic tool is developed for the quick diagnosis of invasive fungal infections, the number of treatments using anti-fungal drugs may decrease significantly, decreasing the potential market for SCY-078.
We believe that a large portion of the treatments using anti-fungal drugs are administered when symptoms of invasive fungal infections are present but a diagnosis of the infection has not yet been made, due to the quick and potentially fatal progression of invasive fungal infections. If a diagnostic tool is developed for the quick diagnosis of invasive fungal infections, then the need to treat in advance of diagnosis of invasive fungal infections may be significantly diminished, which will reduce the potential market for SCY-078 in the event that we are able to obtain FDA approval of SCY-078. Moreover, if a fast and accurate test of the susceptibility of a fungal infection to generically available treatments is developed and widely adopted, the market for SCY-078 may suffer.
If invasive fungi develop resistance to SCY-078, our business will be harmed.
One or more strains of invasive fungi may develop resistance to SCY-078, either because our hypothesis of the mechanism of action is incorrect or because a strain of fungi undergoes some unforeseen genetic mutation that permits it to survive. Since we expect lack of resistance to be a major factor in the commercialization of SCY-078, the development of such resistance would have a major adverse impact on the acceptability and sales of SCY-078.
If we are unable to develop a formulation of SCY-078 that is delivered by intravenous, or IV, therapy SCY-078 may not achieve broad market acceptance and sales will be limited.
Current invasive fungal infection treatment regimens typically involve initial administration of treatments as an IV infusion, with a step down to an oral formulation of the same or a similar medication to complete the course of treatment on an out-patient basis. We believe that providing both the IV and oral formulations will be beneficial to doctors who prefer to start treatment of patients in a hospital setting with an IV therapy and then switch them to an oral formulation of the same medication. We currently have an oral form of SCY-078, and intend to develop an IV formulation. If we are unable to successfully develop and achieve regulatory approval for our IV formulation of SCY-078, or are delayed in developing and obtaining regulatory approval for our IV formulation of SCY-078, our lead product candidate may not achieve, or may be delayed in achieving, broad market acceptance and sales will be limited.
Our product candidates may have undesirable side effects that may delay or prevent marketing approval, or, if approval is received, require them to be taken off the market or otherwise limit their sales.
It is impossible to predict when or if SCY-078 or any other product candidate we may seek to develop will prove effective or safe or will receive marketing approval. Unforeseen side effects from any product candidates could arise either during clinical development or, if approved, after the product has been marketed. For example, the most frequently noted adverse effects reported as associated with SCY-078 treatment in the seven Phase 1 studies of SCY-078 conducted to date were diarrhea, abdominal pain, headache, nausea, fatigue, increased orthostatic heart rate, abnormal GI sounds, vomiting and dizziness. To date there have been two serious adverse events reported in clinical trials of SCY-078: one subject was diagnosed with a metastatic carcinoid tumor which was not considered to be related to SCY-078 by the investigator; and one subject experienced significant liver function test increases which were considered to be related to SCY-078. Preclinical findings in the future could trigger the need to evaluate or monitor for specific potential safety concerns in clinical trials. The results of future clinical trials may show that SCY-078 and any future product candidates we may seek to develop cause undesirable or unacceptable side
17
effects, which could interrupt, delay or halt clinical trials, resulting in delay of, or failure to obtain, marketing approval from the FDA and other regulatory authorities, or may lead us to abandon their development altogether.
Even if SCY-078 or any future product candidate we may seek to develop receives marketing approval, we or others may subsequently identify undesirable or unacceptable side effects caused by these products, in which case:
| Ÿ | regulatory authorities may require the addition of labeling statements, specific warnings, precautions, contraindications or field alerts to physicians and pharmacies; |
| Ÿ | we may be required to change the way the product is administered, conduct additional clinical trials or change the labeling of the product; |
| Ÿ | we may have limitations on how we promote the product; |
| Ÿ | sales of the product may decrease significantly; |
| Ÿ | regulatory authorities may require us to take our approved product off the market; |
| Ÿ | we may be subject to litigation or product liability claims; and |
| Ÿ | our reputation may suffer. |
Any of these events could prevent us or our current or potential future partners from achieving or maintaining market acceptance of the affected product or could substantially increase commercialization costs and expenses, which in turn could delay or prevent us from generating significant revenue from the sale of products.
We have never marketed a drug before, and if we are unable to establish an effective sales force and marketing infrastructure or enter into acceptable third-party sales and marketing or licensing arrangements, we may not be able to successfully commercialize SCY-078 and any future product candidates we may seek to develop.
We currently do not have any sales, distribution and marketing capabilities, the development of which will require substantial resources and will be time consuming. The costs incurred in the development of these capabilities, either internally or through a third-party contract sales organization, would be incurred in advance of any approval of a product candidate. In addition, we may not be able to hire a sales force in the United States that is sufficient in size or has adequate expertise in the medical markets that we intend to target. If we are unable to establish our sales force and marketing capability, our operating results may be adversely affected. In addition, we plan to enter into sales and marketing or licensing arrangements with third parties for international sales of any approved products. If we are unable to enter into or maintain any such arrangements on acceptable terms, or at all, we may be unable to market and sell SCY-078 and any future product candidates we may seek to develop in these markets.
We expect that SCY-078 and any future product candidates we may seek to develop will face competition, and most of our competitors have significantly greater resources than we do.
The pharmaceutical industry is highly competitive, with a number of established, large pharmaceutical companies, as well as many smaller companies. There are many foreign and domestic pharmaceutical companies, biotechnology companies, public and private universities, government agencies and research organizations actively engaged in research and development of products that may target the same markets as SCY-078 and any future product candidates we may seek to develop. We expect any products we develop to compete on the basis of, among other things, product efficacy, price, lack of significant adverse side
18
effects and convenience and ease of treatment. For example, SCY-078 will compete against current leading anti-fungal drugs, including voriconazole from the azole class, caspofungin from the echinocandin class, and liposomal amphotericin B from the polyenes class.
Compared to us, many of our competitors in the anti-fungal market have, and potential competitors for any future product candidates we may seek to develop may have, substantially greater:
| Ÿ | resources, including capital, personnel and technology; |
| Ÿ | research and development capability; |
| Ÿ | clinical trial expertise; |
| Ÿ | regulatory expertise; |
| Ÿ | intellectual property portfolios; |
| Ÿ | expertise in prosecution of intellectual property rights; |
| Ÿ | manufacturing and distribution expertise; and |
| Ÿ | sales and marketing expertise. |
As a result of these factors, our competitors and potential competitors may obtain regulatory approval of their products more rapidly than we do. Our competitors and potential competitors may also develop drugs that are more effective, more widely used and less costly than ours and may also be more successful than us in manufacturing and marketing their products and maintaining compliance with ongoing regulatory compliance.
Reimbursement decisions by third-party payors may have an adverse effect on pricing and market acceptance in the United States. If there is not sufficient reimbursement for our products, it is less likely that our products will be purchased by patients and/or providers.
Successful commercialization of pharmaceutical products usually depends on the availability of adequate coverage and reimbursement from third-party payors, including commercial insurers and, under certain circumstances, federal and state healthcare programs. Patients and/or healthcare providers who purchase drugs generally rely on third-party payors to reimburse all or part of the costs associated with such products. As such, adequate coverage and reimbursement from third-party payors can be essential to new product acceptance and may have an effect on pricing.
Because SCY-078 is not currently commercially available, we do not know the extent to which it will be reimbursed if it is approved by the FDA. If we choose to bring other product candidates to market, they will be subject to similar uncertainty. We believe that SCY-078 and any other product candidates that are brought to market are less likely to be purchased by patients and/or providers if they are not adequately reimbursed by third-party payors.
Furthermore, the market for our product candidates may depend on access to third-party payors’ drug formularies, or lists of medications for which third-party payors provide coverage and reimbursement. Industry competition to be included in such formularies results in downward pricing pressures on pharmaceutical companies. Third-party payors may refuse to include a particular branded drug in their formularies when a competing generic product is available. The adoption of certain payment methodologies by third-party payors may limit our ability to profit from the sale of SCY-078. For example, under Medicare, hospitals are reimbursed under an inpatient prospective payment system. This pricing methodology provides a single payment amount to hospitals based on a given diagnosis-related group. As a result, with respect to Medicare reimbursement for services in the hospital inpatient setting, hospitals could have a financial incentive to use the least expensive drugs for the treatment of invasive fungal infections,
19
particularly the IV formulations of these drugs, as they are typically administered in the hospital, which may significantly impact our ability to charge a premium for SCY-078.
All third-party payors, whether governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs, including mechanisms to encourage the use of generic drugs. Congress has also considered policies to lower the reimbursement formulas in federal and state healthcare programs. Furthermore, coverage of, and reimbursement for, drugs can differ significantly from payor to payor and may require significant time and resources to obtain. In addition, new laws or regulations could impact future coverage and reimbursement.
Healthcare policy changes, including the Affordable Care Act, may have a material adverse effect on us.
In recent years, there have been numerous initiatives on the federal and state levels for comprehensive reforms affecting the payment for, the availability of and reimbursement for healthcare services in the United States, including pharmaceutical products. These initiatives have ranged from proposals to fundamentally change federal and state healthcare reimbursement programs, including providing comprehensive healthcare coverage to the public under governmental funded programs, to minor modifications to existing programs.
In March 2010, Congress enacted the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or the Affordable Care Act. The Affordable Care Act is designed to expand access to affordable health insurance, control healthcare spending, and improve healthcare quality. The law includes provisions to tie Medicare provider reimbursement to healthcare quality and incentives, mandatory compliance programs, enhanced transparency disclosure requirements, increased funding and initiatives to address fraud and abuse, and incentives to state Medicaid programs to expand their coverage and services. It also imposes an annual tax on pharmaceutical manufacturers or importers who sell “branded prescription drugs.” Implementation of the Affordable Care Act is occurring on an ongoing basis, and it is unclear what effect the Affordable Care Act or other state proposals may have on our business.
In addition to the Affordable Care Act, there will continue to be proposals by legislators at both the federal and state levels, regulators and third-party payors to keep drug costs down. Certain of these changes could impose limitations on the prices we will be able to charge for any products that are approved or the amounts of reimbursement available for these products from governmental agencies or third-party payors or may increase the tax requirements for life sciences companies such as ours. We anticipate that the Affordable Care Act and other future healthcare reform proposals could have a material adverse effect on our industry, and may limit our ability to commercialize SCY-078 and any future product candidates we may seek to develop and/or invest in new development.
We expect that a portion of the market for SCY-078 and any other product candidates we may seek to develop will be outside the United States. However, our product candidates may never receive approval or be commercialized outside of the United States.
Before we or any commercial partners can market and commercialize any product candidates outside of the United States, there are numerous and varying regulatory requirements of other countries that will apply. Research and marketing authorization procedures vary among countries and can involve additional product testing and administrative review periods. The marketing authorization process in other countries may include all of the risks detailed above regarding failure to obtain FDA approval in the United States as well as other risks. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country, or identification of potential safety concerns in one country, may have a negative effect on the regulatory process in others. Failure to obtain
20
regulatory approval in other countries or any delay or setback in obtaining such approval could have the same adverse effects detailed above regarding FDA approval in the United States. As described above, such effects include the risks that:
| Ÿ | SCY-078 and any future product candidates we may seek to develop may not generate preclinical or clinical data that are deemed sufficient by regulators in a given jurisdiction; |
| Ÿ | SCY-078 may not be approved for all indications requested, or any indications at all, in a given jurisdiction which could limit the uses of SCY-078 and any future product candidates we may seek to develop and have an adverse effect on product sales and potential royalties; and |
| Ÿ | such approval in a given jurisdiction may be subject to limitations on the indicated uses for which the product may be marketed or require costly post-marketing follow-up studies. |
Foreign countries may have requirements for marketing authorization holders or distributors to have a legal or physical presence in that country, and consideration of and compliance with these requirements may result in additional time and expense before we can pursue or obtain marketing authorization in foreign jurisdictions. If we do receive approval in other countries, we may enter into sales and marketing arrangements with third parties for international sales of any approved products.
Even if SCY-078 or any other future product candidates we may seek to develop receive regulatory approval, we may still face future development and regulatory difficulties.
Even if regulatory approval is obtained for SCY-078 or any other future product candidates we may seek to develop, regulatory authorities may still impose significant restrictions on a product’s indicated uses or marketing or impose ongoing requirements for potentially costly post-approval studies. Given the number of high profile adverse safety events with certain drug products, regulatory authorities may require, as a condition of approval, costly risk evaluation and mitigation strategies, which may include safety surveillance, restricted distribution and use, patient education, enhanced labeling, expedited reporting of certain adverse events, pre-approval of promotional materials and restrictions on direct-to-consumer advertising. For example, any labeling approved for any of our product candidates may include a restriction on the term of its use, or it may not include one or more intended indications. Furthermore, any new legislation addressing drug safety issues could result in delays or increased costs during the period of product development, clinical trials and regulatory review and approval, as well as increased costs to assure compliance with any new post-approval regulatory requirements. Any of these restrictions or requirements could force us or our partners to conduct costly studies.
SCY-078 and any other future product candidates we may seek to develop will also be subject to ongoing regulatory requirements for the packaging, storage, advertising, promotion, record-keeping and submission of safety and other post-market information on the drug. In addition, approved products, manufacturers and manufacturers’ facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing procedures conform to current Good Manufacturing Practices, or cGMP. As such, we and our contract manufacturers, which we will be responsible for overseeing and monitoring for compliance, are subject to continual review and periodic inspections to assess compliance with cGMP. Accordingly, we and others with whom we work must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality control. The FDA may hold us responsible for any deficiencies or noncompliance of our contract manufacturers in relation to SCY-078 and any other future product candidates we may seek to develop. Failure to follow cGMP can result in products being deemed adulterated, which carries significant legal implications. We will also be required to engage in pharmacovigilance activities and report certain adverse reactions and production problems, if any, to the FDA and to comply with certain requirements
21
concerning advertising and promotion for products. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved label. As such, we may not promote products for indications or uses for which they do not have approval. Failure to comply with FDA advertising and promotion standards, which are often subject to interpretation by regulators, may result in a wide range of exposure and liability for us.
If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured or disagrees with the promotion, marketing or labeling of a product, a regulatory agency may impose restrictions on that product or us, including requiring withdrawal of the product from the market. If the manufacturing or marketing of products fail to comply with applicable regulatory requirements, a regulatory agency may:
| Ÿ | issue warning letters; |
| Ÿ | mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners; |
| Ÿ | require us or our partners to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance; |
| Ÿ | impose other civil or criminal penalties; |
| Ÿ | suspend regulatory approval; |
| Ÿ | suspend any ongoing clinical trials; |
| Ÿ | refuse to approve pending applications or supplements to approved applications filed by us, our partners or our potential future partners; |
| Ÿ | impose restrictions on operations, including costly new manufacturing requirements; or |
| Ÿ | seize or detain products or require a product recall. |
Non-compliance may also open a company to potential whistleblower lawsuits, and the potential for liability under the False Claims Act.
Pharmaceutical companies are subject to significant ongoing regulatory obligations and oversight, which may result in significant additional expense and limit our ability to commercialize our products.
We are subject to regulation by other regional, national, state and local agencies, including the Department of Justice, the Office of Inspector General of the U.S. Department of Health and Human Services and other regulatory bodies. Violations of any of the foregoing requirements could result in penalties being assessed against us.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical companies on one hand and prescribers, purchasers and formulary managers on the other. The Affordable Care Act, among other things, clarified that a person or entity need not have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it. In addition, the Affordable Care Act amended the federal civil False Claims Act to provide that a claim, including items
22
or services resulting from a violation of the federal anti-kickback statute, constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. There are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny.
The federal civil False Claims Act prohibits any person from knowingly presenting, or causing to be presented, claims for payment of government funds that are false or fraudulent or knowingly making, or causing to be made, a false record or statement material to a false or fraudulent claim to avoid, decrease or conceal an obligation to pay money to the federal government. Many pharmaceutical and other healthcare companies have been investigated and have reached substantial financial settlements with the federal government under these laws for a variety of alleged marketing activities, including providing free product to customers with the expectation that the customers would bill federal programs for the product; providing consulting fees, grants, free travel, and other benefits to physicians to induce them to prescribe the company’s products; and inflating prices reported to private price publication services, which are used to set drug payment rates under government healthcare programs. Companies have been prosecuted for causing false claims to be submitted because of the marketing of their products for unapproved uses. Pharmaceutical and other healthcare companies have also been prosecuted on other legal theories of Medicare and Medicaid fraud.
The majority of states also have statutes or regulations similar to the federal Anti-Kickback Statute and federal civil False Claims Act, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Some of these states also prohibit certain marketing related activities including the provision of gifts, meals, or other items to certain health care providers. In addition, California, Connecticut, Nevada, and Massachusetts require pharmaceutical companies to implement compliance programs or marketing codes.
Compliance with various federal and state laws is difficult and time consuming, and companies that violate them may face substantial penalties. The potential sanctions include civil monetary penalties, exclusion of a company’s products from reimbursement under government programs, criminal fines and imprisonment. Because of the breadth of these laws and the lack of extensive legal guidance in the form of regulations or court decisions, it is possible that some of our business activities or those of our commercial partners could be subject to challenge under one or more of these laws. Such a challenge could have a material adverse effect on our business and financial condition and growth prospects.
We could become subject to government investigations and related subpoenas. Such subpoenas are often associated with previously filed qui tam actions, or lawsuits filed under seal under the federal civil False Claims Act. Qui tam actions are brought by private plaintiffs suing on behalf of the federal government for alleged federal civil False Claims Act violations. The time and expense associated with responding to such subpoenas, and any related qui tam or other actions, may be extensive, and we cannot predict the results of our review of the responsive documents and underlying facts or the results of such actions. Responding to government investigations, defending any claims raised, and any resulting fines, restitution, damages and penalties, settlement payments or administrative actions, as well as any related actions brought by stockholders or other third parties, could have a material impact on our reputation, business and financial condition and divert the attention of our management from operating our business.
The number and complexity of both federal and state laws continues to increase, and additional governmental resources are being added to enforce these laws and to prosecute companies and individuals who are believed to be violating them. In particular, the Affordable Care Act includes a number of provisions aimed at strengthening the government’s ability to pursue federal Anti-Kickback Statute and federal False Claims Act cases against pharmaceutical manufacturers and other healthcare entities, including substantially increased funding for healthcare fraud enforcement activities, enhanced investigative powers,
23
and amendments to the federal False Claims Act that make it easier for the government and whistleblowers to pursue cases for alleged kickback and false claim violations. Responding to a government investigation or enforcement action would be expensive and time-consuming, and could have a material adverse effect on our business and financial condition and growth prospects.
If we fail to comply with applicable federal, state, or local regulatory requirements, we could be subject to a range of regulatory actions that could affect our ability to commercialize our products and could harm or prevent sales of any affected products that we are able to commercialize, or could substantially increase the costs and expenses of commercializing and marketing our products. Any threatened or actual government enforcement action could also generate adverse publicity and require that we devote substantial resources that could otherwise be used in other aspects of our business.
Regulations, guidelines and recommendations published by various government agencies and organizations may affect the use of SCY-078 and any future product candidates we may seek to develop.
Government agencies may issue regulations and guidelines directly applicable to us, our partners or our potential future partners and our product candidates. In addition, professional societies, practice management groups, private health/science foundations and organizations involved in various diseases from time to time publish guidelines or recommendations to the healthcare and patient communities. These various sorts of recommendations may relate to such matters as product usage, dosage, route of administration and use of related or competing therapies. Changes to these recommendations or other guidelines advocating alternative therapies could result in decreased use of SCY-078 and any future product candidates we may seek to develop, which may adversely affect our results of operations.
Risks Relating to Our Contract Research and Development Services
We are substantially dependent on our agreement with Merial for generation of our revenues, and that agreement expires on December 31, 2014.
We have a research services contract with Merial Limited, or Merial, under which we perform research services for Merial, including the synthesis, purification, and characterization of individual or libraries of compounds, phenotypic screening of compounds, and further testing and optimizing of compounds to support the development of animal health products, which agreement expires on December 31, 2014. Revenues from this contract have accounted for 41% and 44% of our total revenues for the nine months ended September 30, 2013, and year ended December 31, 2012, respectively. If we are not able to extend or replace this contract upon expiration, or if this contract were to terminate prior to December 31, 2014, our ability to generate revenues prior to the commercialization of SCY-078 would be significantly impaired. Merial may also terminate the agreement prior to December 31, 2014 under specified circumstances, including in the event of breach by us of a material obligation if such breach is not remedied after written notice from Merial, or if Merial believes in good faith that we have acted in any way that may subject Merial to liability under anti-corruption laws. During the term and for a period of one year after termination of this agreement for any reason, we cannot provide services to another animal health company using the same intellectual property developed under this agreement, which could also significantly impair our ability to generate revenue from our contract research and development services should this contract terminate.
24
We face potential liability and exposure as a result of the performance of our contract research and development services, and if successful claims are brought against us, we may incur substantial liability, which may exceed the revenues we have received for the performance of our contract research and development services.
To date substantially all of our revenue has been generated from the provision of our contract research and development services. In the event that a regulator asserts that we have conducted activities in a non-compliant manner or a customer asserts that we have conducted our contract research and development services negligently, or otherwise asserts that as a result of the performance of our contract research and development services for that client we have somehow harmed their business or the prospects of their product candidates, we could be subject to litigation, which could divert management’s attention from the operation of our business, including the development of SCY-078. Further, if such litigation is successful, or if we determine that we must settle the litigation, we could be forced to pay substantial damages, which could be more than the revenues that we generated from that customer, as the services that we perform are only a small portion of the development efforts of our customers. Even if we are successful in defending any such claims, we could incur substantial legal costs to do so. Further, publicity of any such litigation or claims could hurt the reputation of our ability to perform contract research and development services, which could cause revenue generated from our contract research and development services to decline. Any such litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
Risks Related to Our Dependence on Third Parties
We are dependent on our existing third-party collaboration with R-Pharm to commercialize SCY-078 in the Russian Federation and certain other countries, and if R-Pharm is not successful in commercializing SCY-078 in those countries, we will lose a significant source of revenue.
We currently have a development license and supply agreement with R-Pharm, CJSC, or R-Pharm, a leading supplier of hospital drugs in Russia, pursuant to which we license to R-Pharm rights to develop and commercialize SCY-078 in the field of human health in Russia and certain smaller non-core markets. R-Pharm will pay us milestone payments upon the achievement of specified milestones, including registration of SCY-078 in a country and upon the achievement of specified levels of sales. In addition, R-Pharm will pay us royalties upon sales of SCY-078 by R-Pharm. We are relying on R-Pharm to commercialize SCY-078 in the countries covered by our agreement with it, and if R-Pharm is not able to commercialize SCY-078 in those countries, or determines not to pursue commercialization of SCY-078 in those countries, we will not receive any milestone or royalty payments under the agreement.
We are dependent on other third-party collaborations to develop and commercialize product candidates we have outlicensed, and if our third-party collaborators are not successful in developing and commercializing product candidates we have outlicensed, we will not receive any revenue from these collaborations.
A significant portion of our strategy is to license to third parties rights to develop and commercialize product candidates we have discovered other than SCY-078, and if these third parties do not perform under our agreements with them, we will not receive any revenue from these collaborations. For example, we currently have a license agreement with Dechra Ltd., or Dechra, pursuant to which we license to Dechra rights to develop and commercialize SCY-641 for use in animal health, and will receive royalties from Dechra on sales of SCY-641. We are relying on Dechra to commercialize SCY-641, and if Dechra is not able to commercialize SCY-641, or determines not to pursue commercialization of SCY-641, we will not receive any royalty payments under the agreement. If our third-party collaborators under this and any future
25
agreements we enter into do not perform under these agreements, we will not receive the benefits we expect under these agreements.
We are dependent on our existing third-party collaborations in animal health to fund additional development opportunities and expect to continue to expend resources in our current collaborations, and if these collaborations fail, then we will lose a significant source of revenues.
We provide contract research and development services in the field of animal health which is a source of significant revenues to us. For example, we have an agreement with Merial, pursuant to which we provide contract research and development services that primarily target parasites, which includes the synthesis, purification, and characterization of individual or libraries of compounds, phenotypic screening of compounds, and further testing and optimizing of compounds for the use of commercializing animal health products. If we are not able to continue to enter into and perform under these services agreements, we will lose the ability to generate significant revenues.
We may not be successful in establishing and maintaining development and commercialization collaborations, which could adversely affect our ability to develop and commercialize product candidates.
Developing pharmaceutical products, conducting clinical trials, obtaining regulatory approval, establishing manufacturing capabilities and marketing approved products is expensive. Consequently, we plan to establish collaborations for development and commercialization of product candidates and research programs. For example, we currently have a development license and supply agreement with R-Pharm, pursuant to which we license to R-Pharm rights to develop and commercialize SCY-078 in the field of human health in Russia and certain smaller non-core markets, and if SCY-078 receives marketing approval, we may enter into additional sales and marketing arrangements with third parties for international sales. If we are unable to enter into any of these arrangements on acceptable terms, or at all, we may be unable to market and sell SCY-078 and any future product candidates we may seek to develop in certain markets. We expect to face competition in seeking appropriate collaborators. Moreover, collaboration arrangements are complex and time consuming to negotiate, document and implement and they may require substantial resources to maintain. We may not be successful in our efforts to establish and implement collaborations or other alternative arrangements for the development of product candidates. When we partner with a third party for development and commercialization of a product candidate, we can expect to relinquish to the third party some or all of the control over the future success of that product candidate. Our collaboration partner may not devote sufficient resources to the commercialization of product candidates or may otherwise fail in their commercialization. The terms of any collaboration or other arrangement that we establish may not be favorable to us. In addition, any collaboration that we enter into may be unsuccessful in the development and commercialization of product candidates. In some cases, we may be responsible for continuing preclinical and initial clinical development of a partnered product candidate or research program, and the payment we receive from our collaboration partner may be insufficient to cover the cost of this development. If we are unable to reach agreements with suitable collaborators for product candidates, we could face increased costs, we may be forced to limit the number of product candidates we can commercially develop or the territories in which we commercialize them and we might fail to commercialize products or programs for which a suitable collaborator cannot be found. If we fail to achieve successful collaborations, our operating results and financial condition will be materially and adversely affected.
We depend on third-party contractors for a substantial portion of our drug development activities and may not be able to control their work as effectively as if we performed these functions ourselves.
We outsource, and intend to continue to outsource, substantial portions of our drug development activities to third-party service providers, including manufacturing and the conduct of our clinical trials and various
26
preclinical studies. Our agreements with third-party service providers and CROs are and will be on a study-by-study basis and typically short-term. In all cases, we expect to be able to terminate the agreements with notice and be responsible for the supplier’s previously incurred costs.
Because we rely on third parties, our internal capacity to perform these functions is limited. Outsourcing these functions involves risk that third parties may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. Even if we outsource activities, in most cases regulators will hold us responsible for the compliance of the activities performed, and hold us responsible for oversight and monitoring of the activities. In addition, the use of third-party service providers requires us to disclose our proprietary information to these parties, which could increase the risk that this information will be misappropriated. There is a limited number of third-party service providers that have the expertise required to achieve our business objectives. Identifying, qualifying and managing performance of third-party service providers can be difficult and time consuming and could cause delays in our development programs. We currently have a small number of employees devoted to clinical development activities, which limits the internal resources we have available to identify and monitor our third-party providers. To the extent we are unable to identify, retain and successfully manage the performance of third-party service providers in the future, our business may be adversely affected.
We have no experience manufacturing product candidates on a large clinical or commercial scale. As a result, we are and will be dependent on third parties for the manufacture of SCY-078 and any future product candidates we may seek to develop, and if we experience problems with any of these third parties, the commercial manufacturing of SCY-078 and any future product candidates we may seek to develop could be delayed.
We have a small number of personnel with experience in drug product manufacturing. If SCY-078 is approved, the inability to manufacture sufficient commercial supplies of the drug product could adversely affect product commercialization. We do not currently have any agreements with third-party manufacturers for the long-term commercial supply of our product candidates, including SCY-078. We may encounter technical difficulties or delays in the transfer of SCY-078 manufacturing on a commercial scale to a third-party manufacturer, or may be unable to enter into agreements for commercial supply with third-party manufacturers, or may be unable to do so on acceptable terms.
We may not be able to establish additional sources of supply for SCY-078 and any future product candidates we may seek to develop. These suppliers are subject to regulatory requirements covering manufacturing, testing, quality control and record keeping relating to product candidates and are also subject to ongoing inspections by the regulatory agencies. Failure by any of our suppliers to comply with applicable regulations may result in long delays and interruptions to our product candidate supply while we seek to secure another supplier that meets all regulatory requirements.
Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured product candidates ourselves, including:
| Ÿ | the possible breach of the manufacturing agreements or violation of regulatory standards by the third parties because of factors beyond our control; and |
| Ÿ | the possibility of termination or nonrenewal of the agreements by the third parties because of our breach of the manufacturing agreement or based on their own business priorities. |
Any of these factors could result in delays or higher costs in connection with our clinical trials, regulatory submissions, required approvals or commercialization of SCY-078 and any future product candidates we may seek to develop.
27
If we fail to establish or lose our relationships with CROs, our drug development efforts could be delayed.
We are substantially dependent on third-party vendors and CROs for preclinical studies and clinical trials related to our drug discovery and development efforts. If we fail to establish or lose our relationship with any one or more of these providers, we could experience a significant delay in both identifying another comparable provider and then contracting for its services, which could adversely affect our development efforts. We may be unable to retain an alternative provider on reasonable terms, or at all. Even if we locate an alternative provider, it is likely that this provider will need additional time to respond to our needs and may not provide the same type or level of services as the original provider. In addition, any contract research organization that we retain will be subject to the FDA’s regulatory requirements and similar foreign standards and we do not have control over compliance with these regulations by these providers. Consequently, if these practices and standards are not adhered to by these providers, the development and commercialization of SCY-078 and any future product candidates we may seek to develop could be delayed, which could severely harm our business and financial condition.
Risks Relating to Our Intellectual Property
We are dependent on Merck for the establishment of our intellectual property rights related to SCY-078, and if Merck has not established our intellectual property rights with sufficient scope to protect SCY-078, we may have limited or no ability to assert exclusive rights to SCY-078.
Under our agreement with Merck, Merck was responsible for establishing the intellectual property rights to SCY-078. As we were not responsible for the establishment of our intellectual property rights to SCY-078, we have less visibility into the strength of our intellectual property rights to SCY-078 than if we had been responsible for the establishment of these rights. If Merck did not establish those rights so they are of sufficient scope to protect SCY-078, then we may not be able to prevent others from using or commercializing SCY-078, and others may be able to assert intellectual property rights in SCY-078 and prevent us from further pursuing the development and commercialization of SCY-078.
It is difficult and costly to protect our proprietary rights, and we may not be able to ensure their protection.
Our commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection of SCY-078 and any future product candidates we may seek to develop and the methods used to manufacture them, as well as successfully defending these patents against third-party challenges. Our ability to stop third parties from making, using, selling, offering to sell or importing SCY-078 and any future product candidates we may seek to develop is dependent upon the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities.
The patent positions of pharmaceutical companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No absolute policy regarding the breadth of claims allowed in pharmaceutical patents has emerged to date in the United States or in many foreign jurisdictions. Changes in either the patent laws or in interpretations of patent laws in the United States and foreign jurisdictions may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be enforced in the patents that we currently own or that may be issued from the applications we have filed or may file in the future or that we have licensed or may license from third parties, including Merck for SCY-078. Further, if any patents we obtain or license are deemed invalid and unenforceable, it could impact our ability to commercialize or license our technology.
28
The degree of future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| Ÿ | others may be able to make compounds that are similar to SCY-078 and any future product candidates we may seek to develop but that are not covered by the claims of our patents; |
| Ÿ | if we encounter delays in our clinical trials, the period of time during which we could market our drug candidates under patent protection would be reduced; |
| Ÿ | we might not have been the first to make the inventions covered by our pending patent applications; |
| Ÿ | we might not have been the first to file patent applications for these inventions; |
| Ÿ | any patents that we obtain may not provide us with any competitive advantages; or |
| Ÿ | the patents of others may have an adverse effect on our business. |
Due to the patent laws of a country, or the decisions of a patent examiner in a country, or our own filing strategies, we may not obtain patent coverage for all of the product candidates or methods involving these candidates in the parent patent application. We plan to pursue divisional patent applications and/or continuation patent applications in the United States and many other countries to obtain claim coverage for inventions that were disclosed but not claimed in the parent patent application, but may not succeed in these efforts.
Composition of matter patents on the active pharmaceutical ingredient are generally considered to be the strongest form of intellectual property protection for pharmaceutical products, as such patents provide protection without regard to any method of use. We cannot be certain that the claims in our patent applications covering composition-of-matter of our drug candidates will be considered patentable by the U.S. Patent and Trademark Office, or USPTO, courts in the United States or by the patent offices and courts in foreign countries. Method of use patents protect the use of a product for the specified method. This type of patent does not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method. Moreover, even if competitors do not actively promote their product for our targeted indications, physicians may prescribe these products “off-label.” Although off-label prescriptions may infringe or contribute to the infringement of method of use patents, the practice is common and such infringement is difficult to prevent or prosecute. Interference proceedings provoked by third parties or brought by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our collaborators or licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our trade secrets or confidential information, particularly in countries where the laws may not protect those rights as fully as in the United States.
There have been numerous changes to the patent laws and proposed changes to the rules of the USPTO, which may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. For example, in September 2011, President Obama signed the America Invents Act that codifies several significant changes to the U.S. patent laws, including, among other things, changing from a “first to invent” to a “first inventor to file” system, limiting where a patent holder may file a patent suit, requiring the apportionment of patent damages, replacing interference or “first to invent” proceedings with derivation actions and creating a post-grant opposition process to challenge patents after they have been issued. The effects of these changes are currently unclear as the USPTO must still
29
implement various regulations, the courts have yet to address any of these provisions and the applicability of the act and new regulations on specific patents discussed herein have not been determined and would need to be reviewed.
Periodic maintenance fees on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
We also may rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, outside scientific collaborators and other advisors may unintentionally or willfully disclose our information to competitors. Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. Our patent applications would not prevent others from discovering and developing new therapies, including therapies for the indications we are targeting. If others seek to develop similar therapies, their research and development efforts may inhibit our ability to conduct research in certain areas and to expand our intellectual property portfolio.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights and we may be unable to enforce or protect our rights to, or use, our technology.
If we choose to go to court to stop another party from using the inventions claimed in any patents we obtain, that individual or company has the right to ask the court to rule that such patents are invalid or should not be enforced against that third party. These lawsuits are expensive and would consume time and resources and divert the attention of managerial and scientific personnel even if we were successful in stopping the infringement of such patents. In addition, there is a risk that the court will decide that such patents are not valid and that we do not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of such patents is upheld, the court will refuse to stop the other party on the grounds that such other party’s activities do not infringe our rights to such patents. In addition, the U.S. Supreme Court has recently modified some tests used by the USPTO in granting patents over the past 20 years, which may decrease the likelihood that we will be able to obtain patents and increase the likelihood of challenge of any patents we obtain or license.
Furthermore, a third party may claim that we or our manufacturing or commercialization partners are using inventions covered by the third party’s patent rights and may go to court to stop us from engaging in our normal operations and activities, including making or selling SCY-078 and any future product candidates we may seek to develop. These lawsuits are costly and could affect our results of operations and divert the attention of managerial and scientific personnel. There is a risk that a court would decide that we or our commercialization partners are infringing the third party’s patents and would order us or our partners to stop the activities covered by the patents. In that event, we or our commercialization partners may not
30
have a viable way around the patent and may need to halt commercialization of the relevant product. In addition, there is a risk that a court will order us or our partners to pay the other party damages for having violated the other party’s patents or obtain one or more licenses from third parties, which may be impossible or require substantial time and expense. We cannot predict whether any license would be available at all or whether it would be available on commercially reasonable terms. Furthermore, even in the absence of litigation, we may need to obtain licenses from third parties to advance our research or allow commercialization of our drug candidates, and we have done so from time to time. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize one or more of our drug candidates, which could harm our business significantly. In the future, we may agree to indemnify our commercial partners against certain intellectual property infringement claims brought by third parties.
Because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
The pharmaceutical and biotechnology industries have produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. For example, we are aware of the existence of other patents relating to the treatment of Hepatitis C Virus which, if we are determined to infringe on those patents, may limit our ability to fully commercialize SCY-635. If we are sued for patent infringement, we would need to demonstrate that our products or methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid, and we may not be able to do this. Proving invalidity is difficult. For example, in the United States, proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents.
Because some patent applications in the United States may be maintained in secrecy until the patents are issued, because patent applications in the United States and many foreign jurisdictions are typically not published until eighteen months after filing and because publications in the scientific literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered by our pending applications. Our competitors may have filed, and may in the future file, patent applications covering technology similar to ours. Any such patent application may have priority over our patent applications, which could further require us to obtain or license rights to issued patents covering such technologies. If another party has filed a U.S. patent application on inventions similar to ours, we may have to participate in an interference proceeding declared by the USPTO to determine priority of invention in the United States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful.
We have received confidential and proprietary information from collaborators, prospective licensees and other third parties. In addition, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed confidential information of these third parties or our employees’ former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial cost and be a distraction to our management and employees.
31
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
Risks Related to Employee Matters and Managing Growth
We may not be able to manage our business effectively if we are unable to attract and retain key personnel.
We may not be able to attract or retain qualified management, finance, scientific and clinical personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses, particularly in the Research Triangle Park area in North Carolina, where we have our offices and research facilities. If we are not able to attract and retain necessary personnel to accomplish our business objectives, we may experience constraints that will significantly impede the achievement of our development objectives, our ability to raise additional capital and our ability to implement our business strategy.
We are highly dependent on the development, regulatory, commercialization and business development expertise of our executive officers and key employees, especially our Chief Executive Officer, Yves Ribeill. If we lose one or more of our executive officers or key employees, our ability to implement our business strategy successfully could be seriously harmed.
We may need to expand our operations and increase the size of our company, and we may experience difficulties in managing growth.
As we advance SCY-078 through preclinical studies, clinical trials and commercialization, we will need to increase our product development, scientific, marketing, sales and administrative headcount to manage these efforts. Our management, personnel and systems currently in place may not be adequate to support this future growth. Our need to effectively manage our operations, growth and various projects requires that we:
| Ÿ | successfully attract and recruit new employees with the expertise and experience we will require; |
| Ÿ | manage our clinical programs effectively, which we anticipate being conducted at numerous clinical sites; |
| Ÿ | develop a marketing and sales infrastructure; and |
| Ÿ | continue to develop our operational, financial and management controls, reporting systems and procedures. |
If we are unable to successfully manage this growth, our business may be adversely affected.
Other Risks Relating to Our Business
We face potential product liability exposure, and if successful claims are brought against us, we may incur substantial liability for a product candidate and may have to limit its commercialization.
The use of product candidates in clinical trials and the sale of any products for which we may obtain marketing approval expose us to the risk of product liability claims. Product liability claims may be brought against us or our partners by participants enrolled in our clinical trials, patients, healthcare providers or others using, administering or selling products. If we cannot successfully defend ourselves against any such claims, we would incur substantial liabilities. Regardless of merit or eventual outcome, product liability claims may result in:
| Ÿ | withdrawal of clinical trial participants; |
32
| Ÿ | termination of clinical trial sites or entire trial programs; |
| Ÿ | costs of related litigation; |
| Ÿ | substantial monetary awards to patients or other claimants; |
| Ÿ | decreased demand for product candidates and loss of revenue; |
| Ÿ | impairment of our business reputation; |
| Ÿ | diversion of management and scientific resources from our business operations; and |
| Ÿ | the inability to commercialize product candidates. |
We have obtained limited product liability insurance coverage for our clinical trials domestically and in selected foreign countries where we are conducting clinical trials. Our coverage is currently limited to $5.0 million per occurrence and $5.0 million in the aggregate per year, as well as additional local country product liability coverage for trials conducted outside of the United States as required by the local country regulations. As such, our insurance coverage may not reimburse us or may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to product liability. We intend to expand our insurance coverage for products to include the sale of commercial products if we obtain marketing approval for product candidates, but we may be unable to obtain commercially reasonable product liability insurance for any products approved for marketing. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our cash necessary to develop SCY-078 and any future product candidates we may seek to develop and adversely affect our business.
Our operations involve hazardous materials, which could subject us to significant liabilities.
Our research and development processes involve the controlled use of hazardous materials, including chemicals. Our operations produce hazardous waste products. We cannot eliminate the risk of accidental contamination or discharge or injury from these materials. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of these materials. We could be subject to civil damages in the event of exposure of individuals to hazardous materials. In addition, claimants may sue us for injury or contamination that results from our use of these materials and our liability may exceed our total assets. We have general liability insurance coverage of up to $1.0 million per occurrence, with an annual aggregate limit of $2.0 million, which excludes pollution liability. This coverage may not be adequate to cover all claims related to our biological or hazardous materials. Furthermore, if we were to be held liable for a claim involving our biological or hazardous materials, this liability could exceed our insurance coverage, if any, and our other financial resources. Compliance with environmental and other laws and regulations may be expensive and current or future regulations may impair our research, development or production efforts. In addition, failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Our insurance policies are expensive and protect us only from some business risks, which will leave us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risk that our business may encounter. Some of the policies we currently maintain include general liability, employment practices liability, property, auto, workers’ compensation, products liability and directors’ and officers’ insurance. We do not know, however,
33
if we will be able to maintain existing insurance with adequate levels of coverage. Any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our cash position and results of operations.
Our research and development activities could be affected or delayed as a result of possible restrictions on animal testing.
Certain laws and regulations require us to test our product candidates on animals before initiating clinical trials involving humans. Animal testing activities have been the subject of controversy and adverse publicity. Animal rights groups and other organizations and individuals have attempted to stop animal testing activities by pressing for legislation and regulation in these areas and by disrupting these activities through protests and other means. To the extent the activities of these groups are successful, our research and development activities may be interrupted, delayed or become more expensive.
Risks Relating to This Offering and Owning Our Common Stock
The market price of our common stock may be highly volatile, and you may not be able to resell your shares at or above the initial public offering price.
Prior to this offering, there has not been a public market for our common stock. Although we expect that our common stock will be approved for listing on the NASDAQ Global Market, if an active trading market for our common stock does not develop following this offering you may not be able to sell your shares quickly or above the initial public offering price. The initial public offering price for the shares will be determined by negotiations between us and the representative of the underwriters and may not be indicative of prices that will prevail in the trading market, and the value of our common stock may decrease from the initial public offering price.
The trading price of our common stock is likely to be volatile. The following factors, in addition to other factors described in this “Risk Factors” section and elsewhere in this prospectus, may have a significant impact on the market price of our common stock:
| Ÿ | the results of our preclinical testing or clinical trials; |
| Ÿ | the ability to obtain additional funding; |
| Ÿ | any delay in filing an NDA or similar foreign applications for SCY-078 and any future product candidate we may seek to develop or any adverse development or perceived adverse development with respect to the FDA’s review of that NDA or a foreign regulator’s review of a similar applications; |
| Ÿ | maintenance of our existing collaborations or ability to enter into new collaborations; |
| Ÿ | our collaboration partners’ election to develop or commercialize product candidates under our collaboration agreements or the termination of any programs under our collaboration agreements; |
| Ÿ | any intellectual property infringement actions in which we or our licensors and collaboration partners may become involved; |
| Ÿ | our ability to successfully develop and commercialize future product candidates; |
| Ÿ | changes in laws or regulations applicable to future products; |
| Ÿ | adverse regulatory decisions; |
| Ÿ | introduction of new products, services or technologies by our competitors; |
| Ÿ | achievement of financial projections we may provide to the public; |
| Ÿ | achievement of the estimates and projections of the investment community; |
34
| Ÿ | the perception of the pharmaceutical industry by the public, legislatures, regulators and the investment community; |
| Ÿ | announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us, our collaboration partners or our competitors; |
| Ÿ | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| Ÿ | legislation or regulation that mandates or encourages the use of generic products; |
| Ÿ | additions or departures of key scientific or management personnel; |
| Ÿ | significant lawsuits, including patent or stockholder litigation; |
| Ÿ | changes in the market valuations of similar companies; |
| Ÿ | general economic and market conditions and overall fluctuations in the U.S. equity markets; |
| Ÿ | sales of our common stock by us, our executive officers and directors or our stockholders in the future; and |
| Ÿ | trading volume of our common stock. |
In addition, companies trading in the stock market in general, and the NASDAQ Global Market in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance.
Our executive officers, directors and principal stockholders own a significant percentage of our stock and will be able to exert significant control over matters submitted to our stockholders for approval.
As of December 31, 2013, our executive officers, directors and stockholders who own more than 5% of our outstanding common stock, together beneficially own shares representing approximately % of our common stock and, upon the closing of this offering, that same group will beneficially own approximately % of our outstanding stock. Therefore, even after this offering, these stockholders will have the ability to influence us through this ownership position. These stockholders may be able to determine all matters requiring stockholder approval. For example, these stockholders, acting together, may be able to control elections of directors, amendments to our organizational documents, or approval of any merger, sale of assets, or other major corporate action. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may believe are in your best interest as one of our stockholders.
We have identified material weaknesses in our internal controls over financial reporting.
Maintaining effective internal controls over financial reporting is necessary for us to produce accurate financial statements on a timely basis. In 2013, we identified material weaknesses in our internal control over financial reporting. Subsequent to the issuance of our 2012 financial statements we determined that a number of items were misstated, as discussed in Note 18 of the notes to our financial statements appearing elsewhere in this prospectus. As a result, previously reported financial information as of and for the years ended December 31, 2012 and 2011, has been restated to correct for these errors. We are currently in the process of remediating these material weaknesses in internal control over financial reporting by, among other things, designing and implementing new procedures and controls. Management continues to devote significant time and attention to remediating these material weaknesses and improving our internal controls, and we expect to continue to incur costs associated with implementing appropriate processes, which could include fees for additional audit and consulting services, which could negatively affect our financial condition and operating results.
35
The requirements associated with being a public company will require significant company resources and management attention.
Following this offering, we will become subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, the listing requirements of the NASDAQ Global Market and other applicable securities rules and regulations. The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition and maintain effective disclosure controls and procedures and internal control over financial reporting. In addition, subsequent rules implemented by the SEC and The NASDAQ Stock Market may also impose various additional requirements on public companies. As a result, we will incur additional legal, accounting and other expenses that we did not incur as a nonpublic company, particularly after we are no longer an “emerging growth company” as defined in the JOBS Act. Further, the need to establish the corporate infrastructure demanded of a public company may divert management’s attention from implementing our growth strategy. We have made, and will continue to make, changes to our corporate governance standards, disclosure controls and financial reporting and accounting systems to meet our reporting obligations. However, the measures we take may not be sufficient to satisfy our obligations as a public company, which could subject us to delisting of our common stock, fines, sanctions and other regulatory action and potentially civil litigation.
Section 404(a) of the Sarbanes-Oxley Act requires annual management assessments of the effectiveness of our internal control over financial reporting, starting with the second annual report that we would expect to file with the SEC, and we will be required to disclose material changes made in our internal controls and procedures on a quarterly basis. Company responsibilities required by the Sarbanes-Oxley Act include establishing corporate oversight and adequate internal control over financial reporting and disclosure controls and procedures. Effective internal controls are necessary for us to produce reliable financial reports and are important to help prevent financial fraud. However, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act until the later of the year following our first annual report required to be filed with the SEC or the date we are no longer an “emerging growth company” as defined in the JOBS Act, because we are taking advantage of the exemptions contained in the JOBS Act.
To build the infrastructure to allow us to assess the effectiveness of our internal control over financial reporting, we will need to hire additional accounting personnel and improve our accounting systems, disclosure policies, procedures and controls, which will be costly and time consuming. If we are unsuccessful in building an appropriate accounting infrastructure, we may not be able to prepare and disclose, in a timely manner, our financial statements and other required disclosures, or comply with existing or new reporting requirements.
If we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We cannot assure you that there will not be material weaknesses in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness in our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations by The NASDAQ Stock Market, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
36
The recently enacted JOBS Act will allow us to postpone the date by which we must comply with some of the laws and regulations intended to protect investors and to reduce the amount of information we provide in our reports filed with the SEC, which could undermine investor confidence in our company and adversely affect the market price of our common stock.
For so long as we remain an “emerging growth company” as defined in the JOBS Act, we may take advantage of certain exemptions from various requirements that are applicable to public companies that are not “emerging growth companies” including:
| Ÿ | the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting; |
| Ÿ | the obligation to provide three years of audited financial statements; |
| Ÿ | the “say on pay” provisions, requiring a non-binding stockholder vote to approve compensation of certain executive officers, and the “say on golden parachute” provisions, requiring a non-binding stockholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations, of the Dodd-Frank Act and some of the disclosure requirements of the Dodd-Frank Act relating to compensation of our chief executive officer; |
| Ÿ | the requirement to provide detailed compensation discussion and analysis in proxy statements and reports filed under the Exchange Act, and instead provide a reduced level of disclosure concerning executive compensation; and |
| Ÿ | any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements. |
We currently intend to take advantage of some of the reduced regulatory and reporting requirements that will be available to us under the JOBS Act so long as we qualify as an “emerging growth company.”
If you purchase shares of our common stock in this offering, you will incur immediate and substantial dilution in the pro forma net tangible book value of your shares.
Investors purchasing common stock in this offering will pay a price per share that substantially exceeds the pro forma net tangible book value per share. As a result, investors purchasing common stock in this offering will incur immediate dilution of $ per share, based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and our pro forma net tangible book value as of December 31, 2013. Further, based on these assumptions, investors purchasing common stock in this offering will contribute approximately % of the total amount invested by stockholders since our inception, but will own only approximately % of the shares of common stock outstanding. For information on how these amounts were calculated, see “Dilution.”
In addition, as of December 31, 2013, options to purchase shares of our common stock, at a weighted average exercise price at December 31, 2013, of $ per share, were outstanding. The exercise of any of these options would result in additional dilution. As a result of the dilution, investors purchasing stock in this offering may receive significantly less than the purchase price paid in this offering, if anything, in the event of our liquidation.
Sales of a substantial number of shares of our common stock in the public market by our existing stockholders could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market, or the perception that these sales might occur, could depress the market price of our common stock and could impair our
37
ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common stock.
All of the shares of common stock sold in this offering will be freely tradable without restrictions or further registration under the Securities Act of 1933, as amended, or the Securities Act, except for any shares held by our affiliates as defined in Rule 144 under the Securities Act or our current stockholders pursuant to lock-up agreements. Substantially all of the remaining shares of common stock outstanding after this offering, based on shares outstanding as of December 31, 2013, will be restricted as a result of securities laws, lock-up agreements or other contractual restrictions that restrict transfers for at least 180 days after the date of this prospectus. RBC Capital Markets, LLC may, in its sole discretion, release all or some portion of the shares subject to lock-up agreements prior to expiration of the lock-up period.
Certain holders of our securities are entitled to rights with respect to the registration of their shares under the Securities Act, subject to the applicable lock-up arrangement described above. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
Future sales and issuances of our common stock or rights to purchase common stock could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. To the extent we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. We may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. These sales may also result in new investors gaining rights superior to our existing stockholders.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the use of the net proceeds from this offering, including for any of the purposes described in the section titled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. The failure of our management to use these funds effectively could have a material adverse effect on our business, cause the market price of our common stock to decline and delay the development of SCY-078 and any future product candidates we may seek to develop. Pending their use, we may invest the net proceeds from this offering in short-term, investment-grade, interest-bearing instruments and U.S. government securities. These investments may not yield a favorable return to our stockholders.
Because we do not intend to declare cash dividends on our shares of common stock in the foreseeable future, stockholders must rely on appreciation of the value of our common stock for any return on their investment.
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends in the future. As a result, we expect that only appreciation of the price of our common stock, if any, will provide a return to investors in this offering for the foreseeable future. Investors seeking cash dividends should not invest in our common stock.
38
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they change their recommendations regarding our common stock adversely, the price of our common stock and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that securities or industry analysts may publish about us, our business, our market or our competitors. If any of the analysts who may cover us change their recommendation regarding our common stock adversely, or provide more favorable relative recommendations about our competitors, the price of our common stock would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the price of our common stock or trading volume to decline.
We may be subject to securities litigation, which is expensive and could divert management attention.
Our share price may be volatile, and in the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Litigation of this type could result in substantial costs and diversion of management’s attention and resources, which could seriously harm our business. Any adverse determination in litigation could also subject us to significant liabilities.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and our bylaws may delay or prevent an acquisition of us, including the ability of our board of directors establish new series of preferred stock and issue shares of these new series, which could be used by our board of directors to oppose a hostile takeover attempt, which some stockholders may believe would be in the best interests of stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management, including the elimination of cumulative voting, inability of our stockholders to call special meetings or take action by written consent, ability of our board of directors to fill board vacancies, and ability of our board of directors to determine the size of the board of directors. In addition, we are subject to Section 203 of the Delaware General Corporation Law, which generally prohibits stockholders owning in excess of 15% of our outstanding voting stock from merging or combining with us. Finally, our charter documents establish advance notice requirements for nominations for election to our board of directors and for proposing matters that can be acted upon at stockholder meetings. Although we believe these provisions together provide for an opportunity to receive higher bids by requiring potential acquirers to negotiate with our board of directors, they would apply even if the offer may be considered beneficial by some stockholders.
39
CAUTIONARY STATEMENT CONCERNING FORWARD-LOOKING STATEMENTS
This prospectus, including the sections titled “Prospectus Summary,” “Risk Factors,” “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Market, Industry and Other Data,” “Business” and “Shares Eligible for Future Sale,” contains forward-looking statements. In some cases you can identify these statements by forward-looking words, such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “goal,” “intend,” “may,” “plan,” “potential,” “seek,” “will,” “would,” or the negative or plural of these words or similar expressions. These forward-looking statements include, but are not limited to, statements concerning the following:
| Ÿ | our ability to successfully develop SCY-078, including an IV formulation of SCY-078; |
| Ÿ | our expectations regarding the benefits we will obtain from the oral form SCY-078 having been designated as a QIDP and the expectation that the IV form will also be designated as a QIDP; |
| Ÿ | our ability to obtain FDA approval of SCY-078; |
| Ÿ | our expectations regarding the devotion of our resources; |
| Ÿ | our expected uses of the net proceeds to us from this offering, and how long they will last; |
| Ÿ | the expected costs of studies and when they will begin; |
| Ÿ | our ability to scale up manufacturing to commercial scale; |
| Ÿ | our reliance on third parties to conduct our clinical studies; |
| Ÿ | our reliance on third-party contract manufacturers to manufacture and supply commercial supplies of SCY-078 for us; |
| Ÿ | our expectations regarding the marketing of SCY-078 should we receive regulatory approval; |
| Ÿ | our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional financing; |
| Ÿ | our financial performance; and |
| Ÿ | developments and projections relating to our competitors or our industry. |
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
You should read this prospectus, and the documents that we reference in this prospectus and have filed with the Securities and Exchange Commission as exhibits to the registration statement of which this
40
prospectus is a part, with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
MARKET, INDUSTRY AND OTHER DATA
We obtained the industry, market and other data throughout this prospectus from our own internal estimates and research, as well as from industry publications and research, surveys and studies conducted by third parties. Industry publications, studies and surveys generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe that each of these studies and publications is reliable, we have not independently verified market and industry data from third-party sources. While we believe our internal company research is reliable and the definitions of our market and industry are appropriate, neither this research nor these definitions have been verified by any independent source.
Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. In some cases, we do not expressly refer to the sources from which this data is derived. In that regard, when we refer to one or more sources of this type of data in any paragraph, you should assume that other data of this type appearing in the same paragraph is derived from the same sources, unless otherwise expressly stated or the context otherwise requires.
41
We estimate that the net proceeds from our issuance and sale of shares of our common stock in this offering will be approximately $ million, or approximately $ million if the underwriters exercise their over-allotment option in full, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the net proceeds from this offering by approximately $ million, assuming that the number of shares we are offering, as set forth on the cover page of this prospectus, remains the same, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of shares in the number of shares we are offering would increase (decrease) the net proceeds to us from this offering by approximately $ million, assuming that the assumed initial public offering price remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
As of December 31, 2013, we had cash and cash equivalents of approximately $ million. We currently estimate that we will use the net proceeds from this offering, together with our cash and cash equivalents as follows:
| Ÿ | approximately $ million for clinical and preclinical costs associated with the completion of Phase 2 and the initiation of Phase 3 trials for our lead product candidate SCY-078; |
| Ÿ | approximately $7.5 million to pay down a portion of our $15.0 million credit facility agreement with HSBC Bank USA, National Association, upon the closing of this offering, with the balance to be paid down as it becomes due; this credit facility has an interest rate of LIBOR plus 0.95% per annum and matures on December 31, 2014; and |
| Ÿ | the balance to fund working capital, capital expenditures and other general corporate purposes. |
This expected use of the net proceeds from this offering and our existing cash and cash equivalents represents our intentions based upon our current plans and business conditions. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development and commercialization efforts and the status of and results from clinical studies, as well as any collaborations that we may enter into with third parties and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment grade, interest bearing instruments and U.S. government securities.
We have never declared or paid, and do not anticipate declaring, or paying in the foreseeable future, any cash dividends on our capital stock. Future determination as to the declaration and payment of dividends, if any, will be at the discretion of our board of directors and will depend on then existing conditions, including our operating results, financial conditions, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant.
42
Dilution is the amount by which the offering price paid by the purchasers of the shares of common stock sold in this offering exceeds the pro forma as adjusted net tangible book value per share of our common stock after this offering. The pro forma net tangible book value of our common stock as of December 31, 2013, was $ million, or $ per share. Pro forma net tangible book value per share represents our total tangible assets less our total liabilities, divided by the number of outstanding shares of common stock, after giving effect to the pro forma adjustments referenced under “Capitalization.”
After giving effect to (a) the pro forma adjustments referenced under “Capitalization” and (b) receipt of the net proceeds from our sale of shares of common stock at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of December 31, 2013, would have been approximately $ million, or $ per share. This represents an immediate increase in pro forma net tangible book value of $ per share to our existing stockholders and an immediate dilution of $ per share to investors purchasing common stock in this offering.
The following table illustrates this dilution on a per share basis to new investors:
| Assumed initial public offering price per share |
$ | |||||||
| Pro forma net tangible book value per share as of December 31, 2013 |
$ | |||||||
| Increase in pro forma net tangible book value per share attributable to new investors purchasing shares in this offering |
||||||||
|
|
|
|||||||
| Pro forma net tangible book value per share after giving effect to this offering |
||||||||
|
|
|
|||||||
| Dilution in pro forma net tangible book value per share to new investors in this offering |
$ | |||||||
|
|
|
If the underwriters’ over-allotment option to purchase additional shares in this offering is exercised in full, the pro forma net tangible book value, as adjusted to give effect to this offering, would be $ per share and the dilution to new investors would be $ per share.
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the pro forma net tangible book value, as adjusted to give effect to this offering but assuming no exercise of the underwriters’ over-allotment option, by $ per share and the dilution to new investors by $ per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
We may also increase or decrease the number of shares we are offering. An increase of shares in the number of shares offered by us would increase our pro forma as adjusted net tangible book value by approximately $ million, or $ per share, and the pro forma dilution per share to investors in this offering by $ per share, assuming that the assumed initial public offering price remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, a decrease of shares in the number of shares offered by us would decrease our pro forma as adjusted net tangible book value by approximately $ million, or $ per share, and the pro forma dilution per share to investors in this offering by $ per share, assuming that the assumed initial public offering price remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma information discussed above is illustrative only and will change based on the actual initial public offering price and other terms of this offering determined at pricing.
43
The table below summarizes as of December 31, 2013, on a pro forma as adjusted basis described above, the number of shares of our common stock, the total consideration, and the average price per share (a) paid to us by our existing stockholders and (b) to be paid by new investors purchasing our common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, before deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| Shares purchased | Total consideration | Average price per share |
||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||
| Existing stockholders |
% | $ | % | $ | ||||||||||||||
| New investors |
% | % | ||||||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
| Total |
100.0 | % | $ | 100.0 | % | |||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) total consideration paid by new investors by $ and increase (decrease) the percent of total consideration paid by new investors by %, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. We may also increase or decrease the number of shares we are offering.
If the underwriters’ over-allotment option to purchase additional shares in this offering is exercised in full, the percentage of shares of our common stock held by existing stockholders will be reduced to % of the total number of shares of our common stock outstanding after this offering, and the number of shares held by new investors will increase to shares, or % of the total number of shares of our common stock outstanding after this offering.
The number of shares of our common stock reflected in the discussion and tables above is based on shares of our common stock outstanding as of December 31, 2013 (including convertible preferred stock on an as-converted basis), and excludes the following:
| Ÿ | shares of our common stock issuable upon the exercise of stock options outstanding at a weighted-average exercise price of $ per share; |
| Ÿ | shares of our common stock reserved for future issuance under our 2009 Stock Option Plan; |
| Ÿ | shares of our common stock reserved for future issuance under our 2014 Equity Incentive Plan; |
| Ÿ | shares of our common stock reserved for future issuance under our 2014 Employee Stock Purchase Plan; and |
| Ÿ | shares of our common stock issuable upon the exercise of convertible preferred stock warrants outstanding and common stock warrants outstanding at a weighted-average exercise price of $ per share. |
To the extent that any outstanding options or warrants are exercised, new options are issued under our stock-based compensation plans or we issue additional shares of common stock in the future, there will be further dilution to investors participating in this offering.
44
The following table sets forth our cash and cash equivalents and capitalization as of December 31, 2013:
| Ÿ | on an actual basis; |
| Ÿ | on a pro forma basis to reflect the filing of our amended and restated certificate of incorporation and the automatic conversion of all outstanding shares of our convertible preferred stock into an aggregate of shares of our common stock and the exercise of all outstanding common stock warrants into an aggregate of shares of our common stock immediately prior to the closing of this offering, and the conversion of all outstanding convertible preferred stock warrants into warrants to purchase an aggregate of shares of our common stock immediately prior to the closing of this offering; and |
| Ÿ | on a pro forma as adjusted basis to further reflect the sale by us of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
You should read this table together with the sections in this prospectus titled “Selected Financial Data,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
| As of December 31, 2013 | ||||||||||||
| Actual | Pro forma | Pro
forma as adjusted(1) |
||||||||||
| (in thousands, except share data) |
||||||||||||
| Cash and cash equivalents |
$ | $ | $ | |||||||||
|
|
|
|
|
|
|
|||||||
| Convertible preferred stock warrant liability |
— | — | ||||||||||
| Convertible preferred stock, $0.001 par value; 30,000,000 shares authorized, shares issued and outstanding, actual; no shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted |
— | — | ||||||||||
|
|
|
|
|
|
|
|||||||
| Stockholders’ (deficit) equity: |
||||||||||||
| Preferred stock, $0.001 par value; shares authorized, no shares issued and outstanding, actual; shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted |
— | — | ||||||||||
| Common stock, $0.001 par value; 70,000,000 shares authorized, shares issued and outstanding, actual; shares authorized, pro forma and pro forma as adjusted; shares issued and outstanding, pro forma; shares issued and outstanding, pro forma as adjusted |
||||||||||||
| Additional paid-in capital |
||||||||||||
| Accumulated deficit |
||||||||||||
| Total stockholders’ (deficit) equity |
||||||||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | $ | $ | |||||||||
|
|
|
|
|
|
|
|||||||
45
| (1) | A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) each of cash and cash equivalents, working capital and total assets by $ and decrease (increase) total stockholders’ deficit by $ , assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase (decrease) of shares that we are offering would increase (decrease) each of pro forma as adjusted cash and cash equivalents, working capital, total assets by approximately $ and decrease stockholders’ deficit by approximately $ , assuming the assumed initial public offering price per share, as set forth on the cover page of this prospectus, remains the same. The pro forma as adjusted information is illustrative only, and we will adjust this information based on the actual initial public offering price, number of shares offered and other terms of this offering determined at pricing. |
The outstanding share information in the table above is based on shares of our common stock outstanding as of December 31, 2013 (including convertible preferred stock on an as-converted basis), and excludes the following:
| Ÿ | shares of our common stock issuable upon the exercise of stock options outstanding at a weighted-average exercise price of $ per share; |
| Ÿ | shares of our common stock reserved for future issuance under our 2009 Stock Option Plan; |
| Ÿ | shares of our common stock reserved for future issuance under our 2014 Equity Incentive Plan; |
| Ÿ | shares of our common stock reserved for future issuance under our 2014 Employee Stock Purchase Plan; |
| Ÿ | shares of our common stock issuable upon the exercise of convertible preferred stock warrants outstanding at a weighted-exercise price of $ per share; and |
| Ÿ | with respect to the actual number of shares outstanding, but not on a pro forma or pro forma as adjusted basis, shares of our common stock issuable upon the exercise of convertible common stock warrants outstanding at a weighted-exercise price of $ per share. |
46
You should read the following selected financial data together with the section of this prospectus titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes included elsewhere in this prospectus. The statement of operations data for the years ended December 31, 2012 and 2011, and the balance sheet data as of December 31, 2012 and 2011, are derived from the audited financial statements that are included elsewhere in this prospectus. The statement of operations data for the nine months ended September 30, 2013 and 2012, and the balance sheet data as of September 30, 2013, are derived from our unaudited financial statements included elsewhere in this prospectus. We have included, in our opinion, all adjustments, consisting only of normal recurring adjustments, that we consider necessary for a fair presentation of the financial information set forth in those financial statements. Our historical results are not necessarily indicative of the results to be expected in the future, and our interim unaudited results are not necessarily indicative of the results to be expected for the full year or any other period.
| Nine Months ended September 30, |
Year Ended December 31, |
|||||||||||||||
| 2013 | 2012 | 2012 | 2011 | |||||||||||||
| (in thousands, except share and per share data) | ||||||||||||||||
| Statement of operations data: |
||||||||||||||||
| Total revenue |
$ | 13,184 | $ | 11,988 | $ | 16,837 | $ | 26,454 | ||||||||
| Cost of revenue |
12,531 | 10,690 | 14,364 | 17,753 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
653 | 1,298 | 2,473 | 8,701 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
3,203 | 6,977 | 8,927 | 11,633 | ||||||||||||
| Selling, general and administrative |
3,150 | 3,742 | 4,742 | 4,980 | ||||||||||||
| Gain on sale of asset |
(988 | ) | (3,412 | ) | (3,412 | ) | — | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
5,365 | 7,307 | 10,257 | 16,613 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(4,712 | ) | (6,009 | ) | (7,784 | ) | (7,912 | ) | ||||||||
| Other (expense) income: |
||||||||||||||||
| Amortization of deferred financing costs and debt discount |
(2,504 | ) | (2,141 | ) | (2,918 | ) | (2,138 | ) | ||||||||
| Interest expense — related party |
(703 | ) | (516 | ) | (747 | ) | (29 | ) | ||||||||
| Interest expense |
(142 | ) | (172 | ) | (225 | ) | (170 | ) | ||||||||
| Derivative fair value adjustment |
(671 | ) | 330 | 185 | 20 | |||||||||||
| Other (expense) income |
— | (8 | ) | 12 | 23 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other expense |
(4,020 | ) | (2,507 | ) | (3,693 | ) | (2,294 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (8,732 | ) | $ | (8,516 | ) | $ | (11,477 | ) | $ | (10,206 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share: |
||||||||||||||||
| Basic and diluted |
$ | (1.27 | ) | $ | (1.30 | ) | $ | (1.73 | ) | $ | (2.53 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted, pro forma(1) |
$ | $ | ||||||||||||||
|
|
|
|
|
|||||||||||||
| Weighted average common shares outstanding: |
||||||||||||||||
| Basic and diluted |
6,852,981 | 6,573,329 | 6,642,837 | 4,034,720 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted, pro forma(1) |
||||||||||||||||
|
|
|
|
|
|||||||||||||
| Stock-based compensation expense included above: |
||||||||||||||||
| Cost of revenue |
$ | 27 | $ | 26 | $ | 103 | $ | 116 | ||||||||
| Research and development |
16 | 10 | 40 | 47 | ||||||||||||
| Selling, general and administrative |
67 | 123 | 215 | 219 | ||||||||||||
47
| (1) | Pro forma basic and diluted net loss per share have been calculated assuming the conversion of all outstanding shares of convertible preferred stock, the conversion of all outstanding convertible promissory notes and the exercise of all warrants issued with our convertible notes into an aggregate of shares of common stock as of the beginning of the applicable period or at the time of issuance, if later. |
| September 30, | December 31, | |||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| (in thousands) | ||||||||||||
| Balance sheet data: |
||||||||||||
| Cash and cash equivalents |
$ | 926 | $ | 2,385 | $ | 3,976 | ||||||
| Working capital (deficit) |
(13,402 | ) | (9,007 | ) | (1,466 | ) | ||||||
| Total assets |
12,146 | 12,118 | 16,585 | |||||||||
| Convertible notes — related party, net of discount |
11,897 | 11,444 | 5,215 | |||||||||
| Long-term debt |
15,000 | 15,000 | 15,000 | |||||||||
| Derivative liability |
2,522 | 683 | 540 | |||||||||
| Convertible preferred stock |
46,086 | 46,086 | 53,486 | |||||||||
| Total stockholders’ deficit |
(70,105 | ) | (65,415 | ) | (61,706 | ) | ||||||
48
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes thereto included elsewhere in this prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this prospectus, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
SCYNEXIS is a pharmaceutical company committed to the discovery, development and commercialization of novel anti-infectives to address significant unmet therapeutic needs. We are developing our lead product candidate, SCY-078, as a novel oral and intravenous (IV) drug for the treatment of serious and life-threatening invasive fungal infections in humans. SCY-078 has been shown to be effective in vitro and in vivo in animal studies against a broad spectrum of medically relevant fungal species, including drug-resistant strains, that account for approximately 90% of invasive fungal infections in the United States and Europe. SCY-078 was shown to be sufficiently safe and well-tolerated in multiple Phase 1 studies to support progression to Phase 2 studies. We anticipate beginning a Phase 2 study in the first half of 2014 with an oral formulation of SCY-078 for the treatment of invasive Candida infection, a common and often fatal invasive fungal infection, and anticipate beginning a Phase 2 study with an IV formulation of SCY-078 in 2015. In addition, we have clinical and preclinical programs based on the use of cyclophilin inhibitors to treat viral diseases. We also provide contract research and development services primarily in the field of animal health, which currently generate substantially all of our revenue.
As a spinout from Aventis in 2000, we began as a chemistry and animal health services company, providing contract research services to third parties. Through the provision of these contract research and development services, we built significant expertise in parasitic infections and drug discovery. Since our formation, we have expanded our animal health capabilities and have discovered a number of proprietary compounds. In June 2004, we entered into an exclusive animal health research collaboration with Merial which included significant milestone and royalty payments. We entered into a revised agreement with Merial effective January 2012 that was non-exclusive, resulting in the ability to provide contract research and development services in the field of animal health for other third parties, but which reduced the amount of research business we receive from Merial. However, we maintain rights to milestones and royalties for products in development under the prior agreement.
The majority of the cash generated by the provision of contract research and development services and the additional capital we have raised has been used to develop proprietary compounds, including SCY-635, our cyclophilin inhibitor compound. In 2013, we exclusively licensed SCY-078 from Merck Sharp & Dohme, or Merck, in the field of human health, and Merck transferred to us the investigational new drug application pending with the U.S. Food and Drug Administration, or the FDA, as well as all data Merck had developed for the compound, plus active pharmaceutical ingredient and tablets. In 2014, Merck assigned the patents to us related to SCY-078 that it had exclusively licensed to us. We are currently seeking a partner for SCY-635 and our cyclophilin inhibitor platform, and are focusing our resources on the development of SCY-078.
Since inception, we have incurred losses associated with development of our proprietary compounds and derived substantially all of our revenue from the provision of our contract research and development
49
services. In the near term, we expect to expend a majority of our capital to develop SCY-078, while continuing to provide our contract research and development services which provide revenues and expert resources. Our net losses were $11.5 million and $10.2 million for the years ended December 31, 2012 and 2011, respectively. Our net losses were $8.7 million and $8.5 million for the nine months ended September 30, 2013 and 2012, respectively. As of September 30, 2013, we had an accumulated deficit of $91.5 million.
We expect to continue to incur significant expenses and operating losses for the foreseeable future, which may fluctuate significantly from quarter to quarter and year to year. We anticipate that our expenses will increase substantially as we:
| Ÿ | continue the development of and seek to obtain regulatory approval for our lead product candidate, SCY-078; |
| Ÿ | prepare for the potential commercialization, manufacturing, and distribution of SCY-078; and |
| Ÿ | add operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts and additional expenses we will incur as a public company. |
Until such time, if ever, that we can generate substantial revenue from product sales, we expect to finance our cash needs through a combination of equity offerings, debt financings, or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. We may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all, which would have a negative impact on our financial condition and could force us to delay, limit, reduce or terminate our research and development programs or commercialization efforts. Failure to receive additional funding could cause us to cease operations, in part or in full.
Collaborations and Licensing Agreements
We have signed a number of licensing and collaboration agreements with partners in human and animal health, including: (1) Merck, a pharmaceutical company, under which we exclusively licensed from Merck its rights to SCY-078 in the field of human health, and agreed to pay Merck milestones upon the occurrence of specified events and will pay tiered royalties based on worldwide sales of SCY-078 when and if it is approved (in 2014, Merck assigned the patents to us related to SCY-078 that it had exclusively licensed to us); (2) Merial, a wholly owned subsidiary of Sanofi, under which we provide animal health research services on a fee for service basis and, with respect to certain product candidates, potential milestones and royalties; (3) R-Pharm, CJSC, a leading supplier of hospital drugs in Russia, granting them exclusive rights in the field of human health to develop and commercialize SCY-078 in Russia and several smaller non-core markets, under which we are entitled to receive potential milestones and royalties; and (4) Dechra Ltd., a UK listed international veterinary pharmaceutical business, granting Dechra rights to SCY-641 in the field of animal health, including dog dry eye, under which we are entitled to receive potential milestones and royalties.
Components of Operating Results
Revenue
To date, we have derived substantially all of our revenue from the provision of our contract research and development services. In addition, we have received upfront and milestone payments in connection with our collaboration and licensing agreements. We expect that any revenue we generate will fluctuate from quarter to quarter as a result of the variability in the amounts of our contract research and development services provided, the achievement of collaboration milestones, and the consummation of new licensing
50
arrangements. We do not expect to generate revenue from product sales for at least the next several years. If we or our collaborators fail to complete the development of product candidates in a timely manner or obtain their regulatory approval, our ability to generate future revenue, and our results of operations and financial position, would be materially adversely affected.
Revenue is recognized when all of the following conditions are met: (1) persuasive evidence of an arrangement exists, (2) rendering of services is complete, (3) fees are fixed or determinable, and (4) collection of fees is reasonably assured.
Cost of Revenue
Cost of revenue primarily consists of salaries and personnel-related costs, including employee benefits and any stock-based compensation. Additional expenses include facilities and equipment costs directly associated with generating revenue, allocated overhead, materials, contracted consultants and other direct costs.
We allocate expenses associated with our facilities, information technology costs, and depreciation and amortization, between cost of revenue and operating expenses. Allocations are based on employee headcount and determined by the nature of work performed.
Research and Development Expense
Research and development expense consists of expenses incurred while performing research and development activities to discover, develop or improve potential product candidates we seek to develop. This includes conducting preclinical studies and clinical trials, manufacturing development efforts and activities related to regulatory filings for product candidates. We recognize research and development expenses as they are incurred. Our research and development expense primarily consists of:
| Ÿ | salaries and personnel-related costs, including benefits and any stock-based compensation for personnel in research and development functions; |
| Ÿ | costs related to executing preclinical and clinical trials; |
| Ÿ | fees paid to consultants and other third parties who support our product candidate development and intellectual property protection; |
| Ÿ | other costs in seeking regulatory approval of our products; and |
| Ÿ | allocated overhead. |
The table below summarizes the total costs incurred for each of our key research and development projects during the periods presented:
| Nine months Ended September 30, |
Years Ended December 31, |
|||||||||||||||
| 2013 | 2012 | 2012 | 2011 | |||||||||||||
| Cyclophilin Inhibitor Platform |
$ | 2,283 | $ | 6,559 | $ | 8,509 | $ | 11,005 | ||||||||
| SCY-078 |
914 | — | — | — | ||||||||||||
| Other |
6 | 418 | 418 | 628 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Research and Development |
$ | 3,203 | $ | 6,977 | $ | 8,927 | $ | 11,633 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Our cyclophilin inhibitor platform and SCY-078 projects were the only key research and development projects during the periods presented. As of September 30, 2013, we have incurred total research and development costs of $63.9 million and $0.9 million, respectively, to develop our cyclophilin inhibitor platform and SCY-078.
51
We plan to increase our research and development expense for the foreseeable future as we continue our effort to develop SCY-078 and to further advance the development of our other product candidates, subject to the availability of additional funding.
The successful development of product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing or costs required to complete the remaining development of any product candidates. This is due to the numerous risks and uncertainties associated with the development of product candidates.
Selling, General and Administrative Expense
Selling, general and administrative expense consists primarily of salaries and personnel-related costs, including employee benefits and any stock-based compensation. This includes personnel in executive, finance, sales, human resources and administrative support functions. Other expenses include facility-related costs not otherwise allocated to cost of revenue or research and development expense, professional fees for auditing, tax and legal services, consulting costs for general and administrative purposes, information systems maintenance and marketing efforts.
We expect that our selling, general and administrative expense will increase as we operate as a public reporting company and develop and commercialize SCY-078. We believe that these increases will likely include increased costs for director and officer liability insurance, costs related to the hiring of additional personnel, and increased fees for outside consultants, lawyers and accountants. We also expect to incur increased costs to comply with corporate governance, internal controls, investor relations, disclosure and similar requirements applicable to public reporting companies.
Gain on Sale of Asset
In May 2012, we sold the rights to internally developed research software to a third-party for $4.5 million. We received an initial payment of $3.5 million in May 2012, and subsequent payments totaling $1.0 million during the nine months ended September, 30, 2013. We recorded these payments as a gain on sale of asset within total operating expenses in each of the respective periods, net of transaction expenses.
Other Income (Expense)
Substantially all of our other income (expense) consists of non-cash costs associated with:
| Ÿ | a related party guarantee of our outstanding credit facility; |
| Ÿ | interest on related party convertible debt; |
| Ÿ | fair value adjustments to our derivative liability for warrants issued in conjunction with the related party convertible debt. |
Interest paid on our outstanding bank debt comprises substantially all of the remaining other income (expense).
In April 2010, we entered into a $15.0 million credit facility agreement with HSBC Bank USA, National Association, or HSBC, which we refer to as the 2010 Credit Agreement or credit facility. This credit facility was guaranteed by a related party. We concluded that the guarantee represents a deemed contribution and recognized the value of the guarantee as deferred financing costs. The value of the guarantee was determined based on the difference between the credit facility’s stated interest rate and the interest rate that would apply if there had been no guarantee from the related party. The value was
52
determined to be $6.3 million at the time the credit facility was established and was amortized over the life of the credit facility. During March 2013, the credit facility and related party guarantee were extended through 2014. At the time of the extension, we concluded that the value of the new guarantee was $3.9 million. This amount was recorded as deferred financing costs and is being amortized through 2014.
From December 2011 through June 2013, we issued convertible promissory notes totaling $12.3 million to related parties. These notes accrued interest at a rate of 8% per year. The purchasers of the convertible notes also received warrants to purchase common stock. The promissory notes, and accrued interest, were converted into preferred stock in December 2013. The warrant fair values were accounted for as a debt discount and amortized over the stated term of the convertibles notes. We concluded that the warrants qualified as a derivative liability and the fair value of the warrants should be adjusted at each reporting period. The amortization of the debt discount is recorded in amortization of deferred financing costs and debt discount and the change in the derivative liability is recorded in derivative fair value adjustment.
Income Tax (Expense) Benefit
Income tax expense consists of U.S. federal and state income taxes. To date, we have not been required to pay U.S. federal income taxes because of our current and accumulated net operating losses.
53
Results of Operations
Comparison of the Nine Months Ended September 30, 2013 and 2012
| Nine Months Ended September 30, | Period-to-Period Change |
|||||||||||||||||||||||
| 2013 | 2012 | |||||||||||||||||||||||
| Amount | Percentage of Revenue |
Amount | Percentage of Revenue |
Amount | Percentage | |||||||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||||||
| Revenue |
$ | 13,184 | 100.0 | % | $ | 11,988 | 100.0 | % | $ | 1,196 | 10.0 | % | ||||||||||||
| Cost of revenue |
12,531 | 95.0 | % | 10,690 | 89.2 | % | 1,841 | 17.2 | % | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Gross profit |
653 | 5.0 | % | 1,298 | 10.8 | % | (645 | ) | (49.7 | )% | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Research and development |
3,203 | 24.3 | % | 6,977 | 58.2 | % | (3,773 | ) | (54.1 | )% | ||||||||||||||
| Selling, general, and administrative |
3,150 | 23.9 | % | 3,742 | 31.2 | % | (592 | ) | (15.8 | )% | ||||||||||||||
| Gain on sale of asset |
(988 | ) | (7.5 | )% | (3,412 | ) | (28.5 | )% | 2,423 | (71.0 | )% | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
5,365 | 40.7 | % | 7,307 | 61.0 | % | (1,942 | ) | (26.6 | )% | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss from operations |
(4,712 | ) | (35.7 | )% | (6,009 | ) | (50.1 | )% | 1,297 | (21.6 | )% | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Other income (expense): |
||||||||||||||||||||||||
| Amortization of deferred financing costs and debt discount |
(2,504 | ) | (19.0 | )% | (2,141 | ) | (17.9 | )% | (363 | ) | 17.0 | % | ||||||||||||
| Interest expense — related party |
(703 | ) | (5.3 | )% | (516 | ) | (4.3 | )% | (187 | ) | 36.2 | % | ||||||||||||
| Interest expense |
(142 | ) | (1.1 | )% | (172 | ) | (1.4 | )% | 30 | (17.4 | )% | |||||||||||||
| Derivative fair value adjustment |
(671 | ) | (5.1 | )% | 330 | 2.8 | % | (1,001 | ) | * | ||||||||||||||
| Other (expense) income |
— | — | % | (8 | ) | (0.1 | )% | 8 | (100 | )% | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total other expense |
(4,020 | ) | (30.5 | )% | (2,507 | ) | (20.9 | )% | (1,513 | ) | 60.4 | % | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss |
$ | (8,732 | ) | (66.2 | )% | $ | (8,516 | ) | (71.0 | )% | $ | (216 | ) | 2.5 | % | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| * | Not applicable or meaningful |
Revenue. Revenue increased by $1.2 million, or 10.0%, to $13.2 million for the nine months ended September 30, 2013 from $12.0 million for the nine months ended September 30, 2012. This increase was primarily attributable to a $1.2 million, or 10.4%, increase in our contract research and development services revenue for the nine months ended September 30, 2013 due to increased services provided as a result of our ability to perform animal research and development services for companies other than Merial.
Cost of Revenue. Cost of revenue increased by $1.8 million, or 17.2%, to $12.5 million for the nine months ended September 30, 2013, from $10.7 million for the nine months ended September 30, 2012. This increase was primarily attributable to a reallocation of headcount from research and development, as we decreased the work on our cyclophilin platform, to cost of revenue to support the growth of our contract research and development services revenue in the nine months ended September 30, 2013. This resulted in increases of $1.6 million in salaries and personnel-related costs and $0.4 million in contracted consultants for the nine months ended September 30, 2013. We anticipate that some or all of this headcount may be reallocated to support the development of SCY-078 as necessary in future periods.
54
Research and Development. Research and development expense decreased by $3.8 million, or 54.1%, to $3.2 million for the nine months ended September 30, 2013, from $7.0 million for the nine months ended September 30, 2012. This decrease was primarily attributable to the reallocation of research and development resources as described above. In addition, we reduced our third-party research and development spending on SCY-635, which we are currently seeking to commercialize with a corporate partner. These events resulted in decreases of $2.3 million in salaries and personnel-related costs and $1.4 million in contracted research and development consultant costs for the nine months ended September 30, 2013. We expect our research and development expense to increase in future periods as we continue to develop SCY-078.
Selling, General and Administrative. Selling, general and administrative expense decreased by $0.6 million, or 15.8%, to $3.1 million for the nine months ended September 30, 2013, from $3.7 million for the nine months ended September 30, 2012. This decrease was primarily attributable to a $0.3 million decrease in administrative expenses principally due to a one-time severance cost of $0.5 million incurred in the nine months ended September 30, 2012 as a result of a reduction in workforce. In addition, the reduction in workforce in 2012 contributed to a $0.3 million decrease in salaries and personnel-related costs during the nine months ended September 30, 2013 as compared to the nine months ended September 30, 2012.
Amortization of Deferred Financing Costs and Debt Discount. Amortization of deferred financing costs and debt discount increased by $0.4 million, or 17.0%, to $2.5 million for the nine months ended September 30, 2013, from $2.1 million for the nine months ended September 30, 2012. This increase was primarily attributable to increases in amortization of finance costs related to a deemed contribution for a guarantee from a related party and a debt discount related to warrants issued with the convertible notes.
Interest Expense — Related Party. Interest expense — related party increased by $0.2 million, or 36.2%, to $0.7 million for the nine months ended September 30, 2013, from $0.5 million for the nine months ended September 30, 2012. This increase was attributable to an increase in indebtedness under convertible notes issued to our investors during the nine months ended September 30, 2013.
55
Comparison of the Years Ended December 31, 2012 and 2011
| Years Ended December 31, | Period-to-Period Change |
|||||||||||||||||||||||
| 2012 | 2011 | |||||||||||||||||||||||
| Amount | Percentage of Revenue |
Amount | Percentage of Revenue |
Amount | Percentage | |||||||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||||||
| Revenues |
$ | 16,837 | 100.0 | % | $ | 26,454 | 100.0 | % | $ | (9,617 | ) | (36.4 | )% | |||||||||||
| Cost of revenues |
14,364 | 85.3 | % | 17,753 | 67.1 | % | (3,389 | ) | (19.1 | )% | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Gross profit |
2,473 | 14.7 | % | 8,701 | 32.9 | % | (6,228 | ) | (71.6 | )% | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Research and development |
8,927 | 53.0 | % | 11,633 | 44.0 | % | (2,706 | ) | (23.3 | )% | ||||||||||||||
| Selling, general, and administrative |
4,742 | 28.2 | % | 4,980 | 18.8 | % | (238 | ) | (4.8 | )% | ||||||||||||||
| Gain on sale of asset |
(3,412 | ) | (20.3 | )% | — | * | (3,412 | ) | * | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
10,257 | 60.9 | % | 16,613 | 62.8 | % | (6,356 | ) | (38.3 | )% | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss from operations |
(7,784 | ) | (46.2 | )% | (7,912 | ) | (29.9 | )% | 128 | (1.6 | )% | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Other income (expense): |
||||||||||||||||||||||||
| Amortization of deferred financing costs and debt discount |
(2,918 | ) | (17.3 | )% | (2,138 | ) | (8.1 | )% | (780 | ) | 36.5 | % | ||||||||||||
| Interest expense — related party |
(747 | ) | (4.4 | )% | |
(29 |
) |
(0.1 | )% | (718 | ) | * | ||||||||||||
| Interest expense |
(225 | ) | (1.3 | )% | (170 | ) | (0.6 | )% | (55 | ) | 32.4 | % | ||||||||||||
| Derivative fair value adjustment |
185 | 1.1 | % | 20 | 0.1 | % | 165 | * | ||||||||||||||||
| Other income |
12 | 0.1 | % | 23 | 0.1 | % | (11 | ) | (47.8 | )% | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total other expense |
(3,693 | ) | (21.9 | )% | (2,294 | ) | (8.7 | )% | (1,399 | ) | 61.0 | % | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss |
$ | (11,477 | ) | (68.2 | )% | $ | (10,206 | ) | (38.6 | )% | $ | (1,271 | ) | 12.5 | % | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| * | Not applicable or meaningful |
Revenue. Revenue decreased by $9.6 million, or 36.4%, to $16.8 million for the year ended December 31, 2012, from $26.5 million for the year ended December 31, 2011. Our contract research and development services revenue decreased $8.5 million, or 34.3% during the year ended December 31, 2012 as compared with the year ended December 31, 2011, primarily due to two factors. First, we revised our agreement with Merial effective January 2012, resulting in reduced revenues under this agreement, and in exchange received the ability to perform contract research and development services in the field of animal health for other partners. Second, competition in the provision of contract research and development services from lower cost providers in Asia, together with internal budget pressures experienced by our customers, reduced demand for our contract research and development services. In addition, we recognized $1.3 million of milestone revenue during the year ended December 31, 2011 related to the achievement of collaboration milestones under our agreement with Merial. This milestone revenue did not recur during the year ended December 31, 2012.
Cost of Revenue. Cost of revenue decreased by $3.4 million, or 19.1%, to $14.4 million for the year ended December 31, 2012, from $17.8 million for the year ended December 31, 2011. This decrease was primarily attributable to reducing customer service and contracted consultant headcount and vacating our
56
satellite laboratory facility. As a result, we experienced a $2.0 million decrease in salaries and personnel-related costs, a $0.6 million decrease in contracted consultant costs and a $0.5 million decrease in facilities and equipment costs for the year ended December 31, 2012.
Research and Development. Research and development expense decreased by $2.7 million, or 23.3%, to $8.9 million for the year ended December 31, 2012, from $11.6 million for the year ended December 31, 2011. This decrease was primarily attributable to a reduction in our third-party research and development spending on SCY-635, which we are currently seeking to commercialize with a corporate partner. This resulted in a decrease of $2.7 million of contracted research and development consultant costs for the year ended December 31, 2012.
Selling, General and Administrative. Selling, general and administrative expense decreased by $0.2 million, or 4.8%, to $4.7 million for the year ended December 31, 2012, from $5.0 million for the year ended December 31, 2011. This decrease was primarily attributable to a favorable change in bad debt expense of $0.7 million related to a $0.4 million decrease in allowance for bad debts and a $0.3 million bad debt recovery during the year ended December 31, 2012. These decreases were partially offset by a one-time severance cost of $0.5 million incurred in 2012 as a result of a reduction in workforce.
Amortization of Deferred Financing Costs and Debt Discount. Amortization of deferred financing costs and debt discount increased by $0.8 million, or 36.5%, to $2.9 million for the year ended December 31, 2012, from $2.1 million for the year ended December 31, 2011. This increase was primarily attributable to increases in amortization of finance costs related to a deemed contribution for a guarantee from a related party and a debt discount related to warrants issued with the convertible notes.
Interest Expense — Related Party. Interest expense — related party increased by $0.7 million from a nominal amount for the year ended December 31, 2011, to $0.7 million for the year ended December 31, 2012. Our convertible notes were issued to our investors in December 2011, and January and May 2012, resulting in a full year of interest expense for the year ended December 31, 2012.
Liquidity and Capital Resources
Sources of Liquidity
Through September 30, 2013, we have funded our operations through revenue from the provision of contract research and development services and $76.8 million from debt and equity issuances. As of September 30, 2013, we had cash and cash equivalents of approximately $0.9 million, compared to $2.4 million and $4.0 million as of December 31, 2012 and 2011, respectively.
We have incurred losses since our inception and, as of September 30, 2013, had an accumulated deficit of $91.5 million. We anticipate that we will continue to incur losses for at least the next several years. We expect that our research and development and selling, general and administrative expenses will continue to increase and, as a result, we will need additional capital to fund our operations, which we may obtain through one or more of equity offerings, debt financings, or other third-party funding, strategic alliances and licensing or collaboration arrangements.
In April 2010, we entered into the 2010 Credit Agreement. The 2010 Credit Agreement comprises a $5.0 million term loan and a $10.0 million revolving credit facility. Borrowings under the 2010 Credit Agreement carry interest at a rate of London InterBank Offered Rate plus 0.95% per annum. The weighted-average interest rate was 1.2%, 1.4% and 1.3% for the nine months ended September 30, 2013, and the years ended December 31, 2012 and 2011, respectively. The full amounts of both the $5.0 million term loan and the $10.0 million revolving credit facility were outstanding as of September 30, 2013, and December 31, 2012 and 2011. All outstanding borrowings under the agreement are guaranteed by a related
57
party with a direct investment in our company, and the 2010 Credit Agreement contains no financial covenants.
In December 2011, we issued convertible notes and warrants to related parties that hold direct investments in our company and received proceeds of $5.5 million. The total principal amount of the convertible notes is $5.5 million and the convertible notes bear interest at a rate of 8% per annum. In January and May of 2012 and in June of 2013, we received $0.2 million and $5.7 million, respectively, from the issuance of additional convertible notes and warrants under the same agreement. In June 2013 we issued convertible notes that bear interest at a rate of 8% per annum to related parties that hold direct investments in our company and received proceeds of $0.9 million. The total principal amount of the convertible notes was $12.3 million as of September 30, 2013.
In December 2013, we issued additional shares of our convertible preferred stock and warrants to purchase shares of our common stock to existing investors in our company and received proceeds of $2.5 million. In connection with this issuance, all principal and interest under our then outstanding convertible notes converted into equity securities that we issued.
Cash Flows
| Nine Months
Ended September 30, |
Years ended December 31, |
|||||||||||||||
| 2013 | 2012 | 2012 | 2011 | |||||||||||||
| (in thousands) | ||||||||||||||||
| Net cash used in operating activities |
$ | (2,979 | ) | $ | (8,656 | ) | $ | (10,596 | ) | $ | (8,958 | ) | ||||
| Net cash provided by (used in) investing activities |
618 | 3,140 | 3,051 | (276 | ) | |||||||||||
| Net cash provided by financing activities |
902 | 5,950 | 5,954 | 12,360 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net (decrease) increase in cash and cash equivalents |
$ | (1,459 | ) | $ | 434 | $ | (1,591 | ) | $ | 3,126 | ||||||
|
|
|
|
|
|
|
|
|
|||||||||
Operating Activities
For the nine months ended September 30, 2013, our net cash used in operating activities of $3.0 million consisted of a net loss of $8.7 million, primarily attributable to our spending on research and development and our selling, general and administrative functions, offset in part by $3.2 million in adjustments for non-cash items and $2.5 million of cash provided by changes in working capital. Adjustments for non-cash items primarily consisted of amortization of deferred financing costs and debt discount of $2.5 million, related to the warrants issued in connection with our convertible debt and a related party guarantee to our credit facility that resulted in a deemed contribution, changes in fair value of our derivative liability of $0.7 million related to the warrants issued in connection with our debt, and depreciation expense of $1.0 million. These were partially offset by a gain on the sale of asset of $1.0 million. The increase in cash resulting from changes in working capital primarily consisted of a $1.6 million increase in deferred revenue, driven primarily by a large advance payment from a customer, a $0.7 million increase in interest payable – related party, which was primarily the result of accumulating interest on outstanding debt obligations, and a $0.3 million decrease in accounts receivable and unbilled services.
For the year ended December 31, 2012, our net cash used in operating activities of $10.6 million consisted of a net loss of $11.5 million, mostly attributable to our spending on research and development, and $0.1 million of cash used to fund changes in working capital, offset by $1.0 million in adjustments for non-
58
cash items. Adjustments for non-cash items primarily consisted of amortization of deferred financing costs and debt discount of $2.9 million, related to the warrants issued in connection with our debt and a related party guarantee to our credit facility that resulted in a deemed contribution, and depreciation expense of $1.5 million. These were partially offset by a gain on the sale of asset of $3.4 million.
For the year ended December 31, 2011, our net cash used in operating activities of $9.0 million consisted of a net loss of $10.2 million, mostly attributable to our spending on research and development, and $3.4 million of cash used to fund changes in working capital, offset by $4.6 million in adjustments for non-cash items. Adjustments for non-cash items primarily consisted of amortization of deferred financing costs and debt discount of $2.1 million, related to the warrants issued in connection with our debt and a related party guarantee to our credit facility that resulted in a deemed contribution, depreciation expense of $1.7 million, change in allowance for bad debts of $0.5 million, and stock-based compensation expense of $0.4 million. The cash used to fund changes in working capital primarily consisted of a decrease in deferred revenue of $1.6 million, which was primarily due to the timing of payments for milestones and related revenue recognition, a decrease in accounts payable and accrued expenses of $1.3 million, resulting from bonus accruals at December 31, 2010 that did not recur at December 31, 2011, and an increase in accounts receivable and unbilled services of $0.9 million. These were partially offset by a decrease in prepaid expenses of $0.4 million.
Investing Activities
For the nine months ended September 30, 2013, net cash provided by investing activities was $0.6 million, which primarily consisted of a gain on sale of internally developed research software of $1.0 million, offset in part by purchases of property and equipment of $0.4 million.
For the year ended December 31, 2012, net cash provided by investing activities was $3.0 million, which primarily consisted of a gain on sale of internally developed research software of $3.4 million, offset in part by property and equipment purchased of $0.4 million.
For the year ended December 31, 2011, net cash used in investing activities was $0.3 million for the purchase of property and equipment.
Financing Activities
For the nine months ended September 30, 2013, net cash provided by financing activities consisted of $0.9 million in proceeds from the issuance of convertible notes.
For the year ended December 31, 2012, net cash provided by financing activities consisted of $6.0 million in proceeds from the issuance of convertible notes.
For the year ended December 31, 2011, net cash provided by financing activities consisted of $7.0 million of borrowings under our revolving credit facility and $5.5 million in proceeds from the issuance of convertible notes. These amounts were partially offset by $0.2 million used to pay debt issuance costs.
Future Funding Requirements
To date, we have not generated any revenue from product sales. We do not know when, or if, we will generate any revenue from product sales. We do not expect to generate significant revenue from product sales unless and until we obtain regulatory approval of and commercialize SCY-078. We do not expect our contract research and development services to support our funding needs associated with the development of SCY-078. In addition, we expect our expenses to increase in connection with our ongoing development activities, particularly as we continue the research, development and clinical trials of, and seek regulatory approval for, product candidates. Upon the closing of this offering, we expect to incur additional costs
59
associated with operating as a public company. In addition, subject to obtaining regulatory approval of product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. We anticipate that we will need substantial additional funding in connection with our continuing operations.
Based upon our current operating plan, we believe that the net proceeds from this offering, together with our existing cash and cash equivalents, will enable us to fund our operating expenses and capital expenditure requirements through at least the first half of 2016. We intend to devote the majority of the net proceeds from this offering to fund our Phase 2 clinical study, planned Phase 3 clinical study and any additional clinical studies necessary to support and to submit an NDA for SCY-078. We have based our estimates on assumptions that may prove to be wrong, and we may use our available capital resources sooner than we currently expect. Because of the numerous risks and uncertainties associated with the development and commercialization of product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures necessary to complete the development of product candidates.
Our future capital requirements will depend on many factors, including:
| Ÿ | the progress, costs, and the clinical development of SCY-078; |
| Ÿ | the outcome, costs and timing of seeking and obtaining FDA and any other regulatory approvals; |
| Ÿ | the ability of product candidates to progress through clinical development successfully; |
| Ÿ | our need to expand our research and development activities; |
| Ÿ | the costs associated with our continuing to support our ability to provide contract research and development services; |
| Ÿ | the costs associated with securing, establishing and maintaining commercialization and manufacturing capabilities; |
| Ÿ | our ability to maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
| Ÿ | our need and ability to hire additional management and scientific and medical personnel; |
| Ÿ | our need to implement additional internal systems and infrastructure, including financial and reporting systems; and |
| Ÿ | the economic and other terms, timing and success of our existing licensing arrangements and any collaboration, licensing or other arrangements into which we may enter in the future. |
Until such time, if ever, as we can generate substantial revenue from product sales, we expect to finance our cash needs through a combination of equity offerings, debt financings, or other third-party funding, cash generated from the provision of contract research and development services, marketing and distribution arrangements, or other collaborations, strategic alliances or licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our common stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through other third-party funding, marketing and distribution arrangements or other
60
collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
Contractual Obligations, Commitments and Contingencies
Our principal commitments consist of obligations under our outstanding long-term debt facilities, non-cancelable leases for our office space and certain equipment, and a purchase commitment for the licensing of the internally developed research software we sold during the year ended December 31, 2012.
The following table summarizes these contractual obligations at December 31, 2012.
| Contractual Obligations |
Total | Less Than 1 Year |
Years 1-3 | Years 4-5 | More Than 5 Years |
|||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Long-term debt: |
||||||||||||||||||||
| Principal payments — related party |
$ | 11,444 | $ | 11,444 | $ | — | $ | — | $ | — | ||||||||||
| Principal payments |
15,000 | — | 15,000 | — | — | |||||||||||||||
| Interest payments — related party |
1,640 | 1,640 | — | — | — | |||||||||||||||
| Interest payments * |
376 | 188 | 188 | — | — | |||||||||||||||
| Operating lease commitments |
6,890 | 919 | 3,290 | 2,378 | 303 | |||||||||||||||
| Purchase commitment |
550 | 400 | 150 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual obligations |
$ | 35,900 | $ | 14,591 | $ | 18,628 | $ | 2,378 | $ | 303 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| * | Interest on our 2010 Credit Agreement is based on a variable interest rate (LIBOR) and is calculated using the interest rate as of the December 31, 2012. |
The following table summarizes these contractual obligations at September 30, 2013. Future events could cause actual payments to differ from these estimates.
| Contractual Obligations |
Total | Less Than 1 Year |
Years 1-3 | Years 4-5 | More Than 5 Years |
|||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Long-term debt: |
||||||||||||||||||||
| Principal payments — related party |
$ | 12,343 | $ | 12,343 | $ | — | $ | — | $ | — | ||||||||||
| Principal payments |
15,000 | — | 15,000 | — | — | |||||||||||||||
| Interest payments — related party |
1,673 | 1,673 | — | — | — | |||||||||||||||
| Interest payments * |
225 | 180 | 45 | — | — | |||||||||||||||
| Operating lease commitments |
6,202 | 1,009 | 3,388 | 1,805 | — | |||||||||||||||
| Purchase commitment |
251 | 251 | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual obligations |
$ | 35,694 | $ | 15,456 | $ | 18,433 | $ | 1,805 | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| * | Interest on our 2010 Credit Agreement is based on a variable interest rate (LIBOR) and is calculated using the interest rate as of the September 30, 2013. |
Subsequent to September 30, 2013, the total outstanding principal and accrued interest balance related to our convertible notes — related party was converted into preferred stock.
The contractual obligations tables do not include any potential milestone payments we may be required to make under our collaboration and licensing agreements as the timing of when these payments will actually be made is uncertain and the payments are contingent upon the initiation and completion of future activities.
61
Off-Balance Sheet Arrangements
During the periods presented we did not have, nor do we currently have, any off-balance sheet arrangements as defined under SEC rules.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with accounting principles generally accepted in the United States, or GAAP. The preparation of our financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of our financial statements, as well as the reported revenues and expenses during the reported periods. We evaluate these estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 3 to our financial statements appearing elsewhere in this prospectus, we believe that the following accounting policies are critical to the process of making significant judgments and estimates in the preparation of our financial statements and understanding and evaluating our reported financial results.
Revenue Recognition and Deferred Revenue
We have historically derived substantially all of our revenue from contract research and development services performed under fee for service arrangements. We have also entered into collaboration and licensing agreements in which multiple elements exist, including the sale of licenses and the provision of services, in exchange for non-refundable upfront payments and consideration as services are performed. Under these arrangements, we are also entitled to receive development milestones and royalties in the form of a designated percentage of product sales. We recognize revenue when there is persuasive evidence of an arrangement, delivery has occurred or we have provided the service, the fees are fixed and determinable and collectability is reasonably assured.
We record amounts received prior to satisfying the above revenue recognition criteria as deferred revenue until all applicable revenue recognition criteria are met.
Stock-Based Compensation
We record the fair value of stock options issued as of the grant date as compensation expense. We recognize compensation expense over the requisite service period, which is equal to the vesting period.
Stock-based compensation expense has been reported in our statements of operations as follows:
| Nine Months Ended September 30, |
Years Ended December 31, |
|||||||||||||||
| 2013 | 2012 | 2012 | 2011 | |||||||||||||
| (in thousands) | ||||||||||||||||
| Cost of revenue |
$ | 27 | $ | 26 | $ | 103 | $ | 116 | ||||||||
| Research and development |
16 | 10 | 40 | 47 | ||||||||||||
| Selling, general and administrative |
67 | 123 | 215 | 219 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 110 | $ | 159 | $ | 358 | $ | 382 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
62
Based upon an assumed initial public offering price of $ per share, the midpoint of the range set forth on the cover of this prospectus, the aggregate intrinsic value of outstanding options to purchase shares of our common stock as of December 31, 2013 was $ million, of which $ million related to vested options and $ million to unvested options.
Determination of the Fair Value of Stock-based Compensation Grants
We calculate the fair value of stock-based compensation arrangements using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the use of subjective assumptions, including volatility of our common stock, the expected term of our stock options, the risk free interest rate for a period that approximates the expected term of our stock options and the fair value of the underlying common stock on the date of grant. In applying these assumptions, we considered the following factors:
| Ÿ | we do not have sufficient history to estimate the volatility of our common stock price. We calculate expected volatility based on reported data for selected reasonably similar publicly traded companies for which the historical information is available. For the purpose of identifying peer companies, we consider characteristics such as industry, length of trading history, similar vesting terms and in-the-money option status. We plan to continue to use the guideline peer group volatility information until the historical volatility of our common stock is relevant to measure expected volatility for future option grants; |
| Ÿ | the assumed dividend yield is based on our expectation of not paying dividends for the foreseeable future; |
| Ÿ | we determine the average expected life of stock options based on the simplified method in accordance with SEC Staff Accounting Bulletin Nos. 107 and 110, as our common stock to date has not been publicly traded. We expect to use the simplified method until we have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term; |
| Ÿ | we determine the risk-free interest rate by reference to implied yields available from U.S. Treasury securities with a remaining term equal to the expected life assumed at the date of grant; and |
| Ÿ | we estimate forfeitures based on our historical analysis of actual stock option forfeitures. |
The assumptions used in the Black-Scholes option-pricing model for the nine months ended September 30, 2013 and 2012 and the years ended December 31, 2012 and 2011, are set forth below:
Employee Stock Options
| Nine Months Ended September 30, |
Years Ended December 31, |
|||||||||||||||
| 2013 | 2012 | 2012 | 2011 | |||||||||||||
| Risk-free interest rate |
1.99 | % | 0.98-1.28 | % | 0.98-1.28 | % | 1.64-2.79 | % | ||||||||
| Expected term (in years) |
6.13-6.49 | 6.13-6.49 | 6.13-6.49 | 5.42-6.49 | ||||||||||||
| Expected volatility |
64.35 | % | 64.10 | % | 64.10 | % | 81.79 | % | ||||||||
| Expected dividend yield |
0 | % | 0 | % | 0 | % | 0 | % | ||||||||
| Forfeiture rate |
5 | % | 5 | % | 5 | % | 5 | % | ||||||||
63
Non-Employee Stock Options
| Nine Months Ended September 30, |
Years Ended December 31, |
|||||||||||||||
| 2013 | 2012 | 2012 | 2011 | |||||||||||||
| Risk-free interest rate |
1.99 | % | 0.98 | % | 0.98-1.28 | % | 2.79 | % | ||||||||
| Expected term (in years) |
5 | 5 | 5 | 5 | ||||||||||||
| Expected volatility |
64.35 | % | 64.35 | % | 64.10 | % | 81.79 | % | ||||||||
| Expected dividend yield |
0 | % | 0 | % | 0 | % | 0 | % | ||||||||
| Forfeiture rate |
5 | % | 5 | % | 5 | % | 5 | % | ||||||||
Determination of the Fair Value of Common Stock on Grant Dates
Historically, we have granted stock options at exercise prices not less than the fair value of our common stock. As there has been no public market for our common stock to date, the estimated fair value of our common stock has been determined by our board of directors. We are a private company with no active public market for our common stock. Therefore, our board of directors has estimated per share fair value of our common stock at each grant date using recently obtained valuations performed in accordance with the guidance outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held Company Equity Securities Issued as Compensation, also known as the Practice Aid. In conducting these valuations, our board of directors considered all objective and subjective factors that it believed to be relevant, including its and management’s best estimate of our business condition, prospects and operating performance at each grant date. In reaching these fair value determinations, our board of directors and management considered a range of objective and subjective factors and assumptions including, among others:
| Ÿ | our results of operations, financial position, status of our research and development efforts, stage of development and business strategy; |
| Ÿ | external market conditions affecting the life sciences and biotechnology industry sectors; |
| Ÿ | the prices at which we sold shares of preferred stock to third-party investors; |
| Ÿ | the superior rights and preferences of the preferred stock relative to our common stock at the time of each grant; |
| Ÿ | our stage of development and business strategy and the material risks related to our business and industry; |
| Ÿ | the valuation of publicly traded companies in the life sciences and biotechnology sectors, as well as recently completed mergers and acquisitions of peer companies; |
| Ÿ | the lack of an active public market for our common and preferred stock; |
| Ÿ | the likelihood of achieving a liquidity event in light of prevailing market conditions, such as an initial public offering or sale of our company; and |
| Ÿ | any recent contemporaneous third-party valuations prepared in accordance with methodologies outlined in the Practice Aid. |
Common Stock Valuation Methodology
We utilize the probability weighted expected return method, or PWERM, approach to allocate value to our common shares. The PWERM approach employs various market, income or cost approach calculations
64
depending on the likelihood of various liquidation scenarios. For each of the various scenarios, an equity value is estimated and the rights and preferences for each stockholder class are considered to allocate the equity value to common stock. The common stock value is then multiplied by a discount factor reflecting the calculated discount rate and the timing of the event. Lastly, the common stock value is multiplied by an estimated probability for each scenario. The probability and timing of each scenario are based on discussions between our board of directors and our management team. Under the PWERM, the value of our common stock is based on five possible future events for our company:
| Ÿ | an initial public offering; |
| Ÿ | an outright strategic sale; |
| Ÿ | a staged strategic sale; |
| Ÿ | remaining a private company; and |
| Ÿ | a sale of our preclinical contract research and development services business. |
Market Approach
The market approach uses similar companies or transactions in the marketplace, referred to as guideline companies. When using the guideline company method of the market approach in determining the fair value of our common stock under the initial public offering scenario, we identified companies similar to our business and used these guideline companies to develop relevant market multiples and ratios. We then applied these market multiples and ratios to our financial forecasts to create an indication of total equity value. In selecting the guideline companies used in our analysis, we applied several criteria, including companies in the life sciences and biotechnology sector, companies displaying economic and financial similarity in certain aspects of primary importance in the eyes of the investing public, and businesses that entail a similar degree of investment risk. When using the similar transaction methodology of the market approach in determining the fair value of our common stock under the strategic merger or sale scenario, we used publicly disclosed data from arm’s-length transactions involving similar companies to develop relationships or value measures between the prices paid for the target companies and the underlying financial performance of those companies. We then applied these value measures to our applicable operating data to create an indication of total equity value.
Income Approach
For the income approach, we used the discounted free cash flow method, which is based on the premise that equity value as of the respective valuation date is equal to the projected future free cash flows and expected terminal value of the business, discounted by a required rate of return that investors would demand given the risks of ownership and the risks associated with achieving the stream of projected future free cash flows.
Cost Approach
We did not use the cost approach, which adjusts a company’s significant tangible assets to market value, in our valuations because our value relates primarily to the intangible assets that are more appropriately valued using the market or income approaches.
65
The following table summarizes by grant date the number of shares of common stock subject to stock options granted from January 1, 2012 through the date of this prospectus, as well as the associated per share exercise price and the estimated fair value per share of our common stock on the grant date.
| Grant Date |
Number of Shares Underlying Options Granted |
Exercise Price per Share |
Estimated Fair Value per Share |
|||||||||
| July 12, 2012 |
177,538 | $ | 1.20 | $ | 1.20 | |||||||
| October 25, 2012 |
227,700 | $ | 1.20 | $ | 0.90 | |||||||
| July 11, 2013 |
32,218 | $ | 1.00 | $ | 1.00 | |||||||
| December 20, 2013 |
203,000 | $ | 2.70 | * | ||||||||
| January 16, 2014 |
951,393 | $ | 2.70 | * | ||||||||
| * | We are in the process of obtaining a valuation report that would provide an indication of the fair value of our common stock as of the date of these stock option grants. |
Significant factors contributing to the determination of common stock fair value at the date of each grant beginning in fiscal year 2012 were as follows:
July and October 2012 Stock Option Grants. Our board of directors granted options to purchase 177,538 shares of common stock with an exercise price per share of $1.20 on July 12, 2012. In estimating the fair value of our common stock to set the exercise price of these options as of July 12, 2012, our board of directors reviewed and considered a valuation report for our common stock as of December 31, 2011. The valuation report reflected a fair value for our common stock of $1.20 as of December 31, 2011. Our board of directors determined that there were no significant factors affecting the value of our common stock that had occurred between December 31, 2011 and July 12, 2012.
Less than four months later, on October 25, 2012, when our results were similar to prior months, our board of directors granted options to purchase 227,700 shares of common stock with an exercise price per share of $1.20. Little had changed in our business since the last stock option grant date and the overall market conditions had not changed significantly. Therefore, our board of directors determined that the estimated fair value of common stock had not changed since the July 12, 2012 grants.
The primary valuation considerations were:
| Ÿ | the liquidity event scenario probabilities and valuation methods used for determining the fair value of our common stock, which were as follows: |
| Scenario |
Probability | Valuation Method | ||||||
| Initial public offering |
0 | % | N/A | |||||
| Outright strategic sale |
15 | % | Market | |||||
| Staged strategic sale |
30 | % | Market | |||||
| Remain a private company |
25 | % | Income/Market | |||||
| Sale of contract research and development services business |
30 | % | Market | |||||
Our board of directors determined that the general initial public offering market, specifically for small life sciences and biotechnology companies, was not very strong and that we lacked a viable product candidate in our pipeline that we could reasonably expect to commercialize. Therefore, it was unlikely that we would be able to successfully complete an initial public offering in the foreseeable future and thus assigned a probability of zero to this scenario. Our board of directors considered a staged strategic sale with an upfront cash payment, followed by a contingent payment based on the success of development efforts within a stipulated timeframe, to be more probable than an outright sale and thus
66
assigned a 30% probability to this scenario compared to a 15% probability to the outright strategic sale scenario. Our board of directors considered remaining private to be possible but slightly less likely than a staged strategic sale, resulting in this scenario being assigned a 25% probability. Lastly, our board of directors considered the sale of our contract research and development services business followed by a staged sale of the remaining business to be of equal likelihood as a staged strategic sale scenario, thus assigning a 30% probability to this scenario;
| Ÿ | a discount rate of 31.2%, based on our estimated cost of capital; and |
| Ÿ | a lack of marketability discount of 25%. |
In preparation for this filing, we received a valuation of our common stock as of September 30, 2012 for the sole purpose of determining the fair value of our derivative liability for re-measurement purposes. This valuation report reflected a fair value for our common stock of $0.90 as of September 30, 2012, which is lower than the exercise price per share of $1.20 for our October 25, 2012 stock option grants.
July 2013 Stock Option Grants. Our board of directors granted options to purchase 32,218 shares of common stock with an exercise price per share of $1.00 on July 11, 2013. In estimating the fair value of our common stock to set the exercise price of such options as of July 11, 2013, our board of directors reviewed and considered a valuation report for our common stock as of December 31, 2012. The valuation report reflected a fair value for our common stock of $1.00 as of December 31, 2012. Our board of directors determined that there were no significant factors affecting the value of our common stock that had occurred between December 31, 2012 and July 11, 2013.
The primary valuation considerations were:
| Ÿ | the liquidity event scenario probabilities and valuation methods used for determining the fair value of our common stock, which were as follows: |
| Scenario |
Probability | Valuation Method | ||||||
| Initial public offering |
10 | % | Market | |||||
| Outright strategic sale |
15 | % | Market | |||||
| Staged strategic sale |
30 | % | Market | |||||
| Remain a private company |
20 | % | Income/Market | |||||
| Sale of contract research and development services business |
25 | % | Market | |||||
Our board of directors determined that the initial public offering market was improving, particularly within the life sciences and biotechnology sector and for companies of similar size and stage as us, and believed an initial public offering in mid-2014 was a possibility, thus assigning a probability of 10% to this scenario. Our board of directors considered remaining private to be possible but slightly less likely than the previous valuation given the uptick in initial public offering activity, resulting in this scenario being assigned a 20% probability. Similarly, our board of directors considered the sale of our contract research and development services business followed by a staged sale of the remaining business to be slightly less likely than a staged strategic sale scenario, thus assigning a 25% probability to this scenario;
| Ÿ | a discount rate of 30.2%, based on our estimated cost of capital; and |
| Ÿ | a lack of marketability discount of 25%. |
December 2013 and January 2014 Stock Option Grants. Our board of directors granted options to purchase 203,000 and 951,393 shares of common stock on December 20, 2013 and January 16, 2014, respectively, with an exercise price per share of $2.70. In setting the exercise price of these options as of the respective grant dates, our board of directors reviewed and considered a valuation report for our common stock as of September 30, 2013. The valuation report reflected a fair value for our common stock of $1.45
67
as of September 30, 2013. Our board of directors determined that there were significant factors which occurred between September 30, 2013 and the respective stock option grant dates that increased the fair value of our common stock, specifically:
| Ÿ | the board of directors made a decision to proceed with an initial public offering of our common stock; and |
| Ÿ | we selected a lead underwriter for the initial public offering. |
Having considered these factors, our board of directors set the exercise price per share at $2.70. We are in the process of obtaining a valuation that will consider these factors and, therefore, provide an indication of the fair value of our common stock as of the date of these stock option grants.
The primary valuation considerations as of September 30, 2013 were:
| Ÿ | the liquidity event scenario probabilities and valuation methods used for determining the fair value of our common stock, which were as follows: |
| Scenario |
Probability | Valuation Method | ||||||
| Initial public offering |
40 | % | Market | |||||
| Outright strategic sale |
10 | % | Market | |||||
| Staged strategic sale |
30 | % | Market | |||||
| Remain a private company |
10 | % | Income/Market | |||||
| Sale of contract research and development services business |
10 | % | Market | |||||
We determined that the initial public offering market within the life sciences and biotechnology sector and for companies of similar size and stage as us continued to be strong. Further, as a result of our meeting with the FDA in September 2013 we discovered that we may significantly reduce the time and expense associated with progressing SCY-078 through Phase 2 and Phase 3 studies. Therefore, we believed an initial public offering in the first quarter of 2014 was a strong possibility and, thus, assigned a probability of 40% to this scenario. Because a significant part of the value of the company is attributable to drugs in development, our board of directors considered a staged strategic sale the most probable outcome if an initial public offering did not occur, continuing to assign it a probability of 30%. We considered an outright strategic sale, remaining private, or a sale of our contract research and development services business followed by a staged sale of the remaining business to be possible but slightly less likely than the previous valuation given the higher probability of an initial public offering, resulting in each of these scenarios being assigned a probability of 10%;
| Ÿ | a lower discount rate of 25.3% due to reduced uncertainties associated with the operating forecast; and |
| Ÿ | a lower lack of marketability discount of 10% due to a higher probability of a liquidity event in the next six months. |
Determination of the Fair Value of Common Stock Warrants on Issuance Dates
We have issued warrants to purchase our common stock in connection with the issuances of convertible notes and the issuance of Series D-2 convertible preferred stock. We calculated the fair value of common stock warrants at their intrinsic value, which is the estimated fair value of the common stock less the exercise price for the warrant. At the date of issuance, the fair value of the warrants is recognized as a debt discount to the convertible notes, which is amortized to expense over the stated term of the related
68
notes, and as a long-term derivative liability, which is adjusted at each reporting period to reflect its fair value calculated based on the latest fair value of our common stock.
We issued common stock warrants with nominal exercise prices. The following table summarizes the number of shares of common stock subject to warrants granted from January 1, 2012 through the date of this prospectus, as well as the associated per share exercise price, and the estimated fair value per share of our common stock at the grant date:
| Issuance Date |
Number of Shares Underlying Common Stock Warrants Issued |
Exercise Price per Share |
Estimated Fair Value per Share |
|||||||||
| January 27, 2012 |
10,287 | $ | 0.01 | $ | 1.20 | |||||||
| May 15, 2012 |
265,360 | $ | 0.01 | $ | 1.20 | |||||||
| December 11, 2013 |
4,705,170 | $ | 0.01 | * | ||||||||
| * | We are in the process of obtaining a valuation that will provide an indication of the fair value of our common stock as of the date of this common stock warrant issuance. |
January 2012 Common Stock Warrant Issuance. On January 27, 2012, we issued warrants to purchase 10,287 shares of our common stock in connection with an issuance of convertible notes. In estimating the fair value of our common stock warrants at the issuance date, we reviewed and considered the valuation report for our common stock as of December 31, 2011 that reflected a fair value for our common stock of $1.20 per share. We determined that there were no significant factors affecting the value of our common stock that had occurred between December 31, 2011 and January 27, 2012. The primary valuation considerations are discussed in the section of this prospectus titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Significant Judgments and Estimates—Stock-Based Compensation—Determination of the Fair Value of Common Stock on Grant Dates.”
May 2012 Common Stock Warrant Issuance. On May 15, 2012, we issued warrants to purchase 265,360 shares of our common stock in connection with an issuance of convertible notes. In estimating the fair value of our common stock warrants at the issuance date, we reviewed and considered the valuation report for our common stock as of December 31, 2011 that reflected a fair value for our common stock of $1.20 per share. We determined that there were no significant factors affecting the value of our common stock that had occurred between December 31, 2011 and May 15, 2012. The primary valuation considerations are discussed in the section of this prospectus titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Significant Judgments and Estimates—Stock-Based Compensation—Determination of the Fair Value of Common Stock on Grant Dates.”
December 2013 Common Stock Warrant Issuances. On December 11, 2013, holders of the June 2013 convertible notes, upon their request under the terms of the convertible notes agreement, received warrants to purchase 1,815,385 shares of our common stock. In addition, we also issued warrants to purchase 1,785,712 shares of our common stock in connection with the issuance of Series D-2 convertible preferred stock on December 11, 2013.
We are in the process of obtaining a valuation that will provide an indication of the fair value of our common stock as of the date of this common stock warrant issuance.
69
Issuance of Series D-1 and D-2 Convertible Preferred Stock
On December 11, 2013, we entered into an agreement to sell 1,785,712 shares of Series D-2 convertible preferred stock at $1.40 per share for an aggregate price of $2.5 million.
Concurrent with the sale, our board of directors and the holders of the convertible notes amended the terms of the outstanding convertible notes. Under the amendment, the outstanding principal and accrued interest balance became convertible into Series D-1 and Series D-2 convertible preferred stock at a conversion price of $1.40 per share. Upon approval of this amendment, holders of the convertible notes elected to convert their outstanding balances and received 6,054,255 shares of Series D-1 convertible preferred stock and 3,956,985 shares of Series D-2 convertible preferred stock.
We are in the process of obtaining a valuation that will provide an indication of the fair value of our Series D-1 and D-2 convertible preferred stock as of the date of these issuances.
Deferred Financing Costs
We incur financing costs associated with issuing our debt facilities and recognize these costs in our balance sheet as noncurrent assets. We amortize our deferred financing costs over the life of the related debt.
Our most significant financing cost incurred to date is associated with our credit facility entered into in April 2010 and extended in March 2013. The credit facility was guaranteed by a related party. We concluded that the guarantee represents a deemed contribution and recognized the fair value of the guarantee as deferred financing costs. We determined the value of the guarantee based on the difference between the credit facility’s stated interest rate and the interest rate that would apply had there been no guarantee from the related party. The value was determined to be $6.3 million at the time the credit facility was established and was amortized over the life of the credit facility. During March 2013, the credit facility and related party guarantee were extended through 2014. At the time of the extension, we concluded that the value of the new guarantee was $3.9 million. This amount was recorded as deferred financing costs and is being amortized through 2014.
Fair Value of Financial Instruments
We have common and preferred stock warrants that meet the definition of derivative financial instruments and are accounted for as derivatives. The fair value of these warrant derivatives is based on a valuation of our common stock at each reporting period. In order to determine the fair value of our common stock, we use a probability-weighted expected return method, or PWERM. Significant inputs for the PWERM included an estimate of our equity value, a weighted average cost of capital, and an estimated probability and timing for each valuation scenario.
Upon exercise of the warrants, we will adjust the derivative liability to fair value with any changes recorded in other income (expense). At such time, the derivative liability will also be reclassified to additional paid-in capital, and no further revaluations will be necessary.
Utilization of Net Operating Loss Carryforwards
As of December 31, 2012, we had federal net operating loss, or NOL, carryforwards of approximately $64.8 million, North Carolina net economic loss, or NEL, carryforwards of approximately $69.2 million, and Pennsylvania NOL carryforwards of approximately $0.1 million. The federal NOL, North Carolina NEL, and Pennsylvania NOL carryforwards begin to expire in 2020, 2015, and 2022, respectively. As of September 30, 2013, we had federal research and development credit carryforwards of $2.1 million and North Carolina credit carryforwards of $0.1 million, which begin to expire in 2020 and 2015, respectively.
70
In accordance with Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a change in equity ownership of greater than 50% within a three-year period results in an annual limitation on a company’s ability to utilize its NOL carryforwards created during the tax periods prior to the change in ownership. We have determined that we have experienced Section 382 ownership changes in the past and a portion of our NOL carryforwards are subject to an annual limitation under Section 382 of the Code. If we experience a Section 382 ownership change in connection with this offering or as a result of future changes in our stock ownership, the tax benefits related to the NOL carryforwards may be further limited or lost.
Recent Accounting Pronouncements
We anticipate that the adoption of recently issued accounting standards will have no impact on our financial condition, results of operations, or disclosures.
Quantitative and Qualitative Disclosure about Market Risk
Interest Rate Sensitivity
Our cash and cash equivalents as of September 30, 2013 consisted of cash maintained in several FDIC insured operating accounts. Our primary exposure to market risk for our cash and cash equivalents is interest income sensitivity, which is affected by changes in the general level of U.S interest rates. However, we do not believe a sudden change in the interest rates would have a material impact on our financial condition or results of operations.
We are subject to interest rate risk in connection with borrowing under our credit agreement, which comprises a $5.0 million term loan and a $10.0 million revolving credit facility. Borrowings under the agreement carry interest at a rate of LIBOR plus 0.95% per annum. Any borrowings under this agreement are at a variable rate and, as a result, increases in market interest rates would generally result in increased interest expense on our outstanding borrowings. As of September 30, 2013, we had $15.0 million outstanding under the agreement. As a result, each change of one percentage point in interest rates would result in an approximate $0.2 million change in our annual interest expense on our outstanding borrowings.
Inflation
We do not believe that inflation and changing prices has had a significant impact on our business, financial condition or results of operations for any periods presented.
71
Overview
SCYNEXIS is a pharmaceutical company committed to the discovery, development and commercialization of novel anti-infectives to address significant unmet therapeutic needs. We are developing our lead product candidate, SCY-078, as a novel oral and intravenous (IV) drug for the treatment of serious and life-threatening invasive fungal infections in humans. SCY-078 has been shown to be effective in vitro and in vivo in animal studies against a broad spectrum of medically relevant fungal species, including drug-resistant strains, that account for approximately 85% of invasive fungal infections in the United States and Europe. SCY-078 was shown to be sufficiently safe and well-tolerated in multiple Phase 1 studies to support progression to Phase 2 studies. We anticipate beginning a Phase 2 study in the first half of 2014 with an oral formulation of SCY-078 for the treatment of invasive Candida infection, a common and often fatal invasive fungal infection, and anticipate beginning a Phase 2 study with an IV formulation of SCY-078 in 2015. In addition, we have clinical and preclinical programs based on the use of cyclophilin inhibitors to treat viral diseases. We also provide contract research and development services primarily in the field of animal health, which currently generate substantially all of our revenue.
The worldwide market for prescription anti-fungal therapeutics, where we will target SCY-078, was expected to be $5.9 billion in 2011 and is forecasted to grow to $6.5 billion in 2016. Incidence rates of confirmed infection by Candida and Aspergillus species indicate that these two pathogens cause over 450,000 invasive fungal infections each year. The rapid progression of the disease and the high mortality rates associated with invasive fungal infections often result in treatments being administered in unconfirmed cases or as a preventative measure, and we estimate that the total cases treated to be approximately three to four times the number of confirmed cases. Also, there is increasing use of drugs that suppress the immune system, such as chemotherapies or drugs for auto-immune disease and transplantation, which has led to an increased rate of invasive fungal infections. Furthermore, the limited number of anti-fungal drug classes, consisting of azoles, echinocandins and polyenes, and their overuse, has led to increased numbers of, and infections with, drug-resistant strains. The resulting pattern of infection, followed by treatment, followed by the development of resistance, followed by more infections is familiar to the medical community, as it has faced these same issues with multi-drug resistant bacterial infections such as methicillin-resistant Staphylococcus aureus, commonly known as MRSA.
SCY-078 represents a new chemical class of drugs designed to block an established target in infectious fungi. We have conducted studies of SCY-078 using animal models that were used in the development of previously approved anti-fungal drugs where these models were proven to be predictive of efficacy in humans. Using these well-established animal models, SCY-078 was shown to be highly active against Candida and Aspergillus. SCY-078 has shown potent in vitro activity against a large collection of medically relevant strains of Candida and Aspergillus, including multi-drug resistant strains that have been isolated from infected patients. Across seven Phase 1 studies, which included over 100 healthy human volunteers, SCY-078 achieved sustained blood concentrations at levels believed to be clinically relevant and was sufficiently safe and well tolerated to support progression to Phase 2 studies. We are developing both an IV and oral formulation of SCY-078 because patients are typically prescribed IV treatment in hospitals, and then are switched, or “stepped down,” to oral formulations when the patient shows sufficient improvement of symptoms. The availability of SCY-078 in both oral and IV formulations would allow patients to remain within the same drug class and potentially be discharged from the hospital sooner.
As the next step in the development of SCY-078, we plan to conduct a randomized Phase 2 study, scheduled to commence in the first half of 2014. This will be a three arm study comparing two doses of SCY-078 to current standard of care in patients with invasive Candida infections, including patients infected with species of Candida which are resistant to azoles, patients previously treated with azole
72
therapy, and treatment-naïve patients. We also intend to initiate a Phase 2 study with an IV formulation of SCY-078 in the first half of 2015 in patients with invasive Candida infections. We anticipate this study will include the option of stepping patients down from IV to oral SCY-078.
If approved, we intend to market SCY-078 to hospitals and major medical centers, where physicians specializing in critical care, infectious disease specialists, and physicians treating immune-compromised or immune-suppressed patients, such as oncologists and those performing solid organ transplants and stem cell transplants, are likely to be found and where invasive fungal infections are more prevalent.
Despite the increasing availability of generic azole drugs and the eventual availability of generic echinocandin drugs, we believe SCY-078, once commercialized, will be able to achieve premium branded pricing comparable to that of the top selling branded hospital-based antibiotics. We believe we can achieve attractive premium pricing even with the increasing availability of generic drugs. We anticipate positioning SCY-078 for use in patients infected with multi-drug resistant strains and as an alternative to echinocandins.
| Ÿ | Drug resistant strains. There are many invasive fungal strains resistant to azole drugs. High rates of morbidity and mortality, and extended hospital stays associated with infections from drug resistant strains, will make a strong argument for use of a premium-priced anti-fungal drug which is effective against these resistant strains. |
| Ÿ | Alternative to echinocandins. Physicians are reluctant to prescribe azoles in hospitals where azole resistance is prevalent, as an ineffective course of therapy can compromise the patient’s survival. Thus, in these settings, physicians often prescribe echinocandins; but echinocandins are only available in IV formulation. Subsequent step down to an oral azole to allow release from the hospital risks emergence of an azole resistant infection, leading some physicians to keep patients on IV echinocandins for the full course of therapy. If successfully developed, SCY-078 would provide an attractive alternative to echinocandin therapy by offering an IV-to-oral step-down within a single therapeutic class, thereby facilitating earlier discharge from the hospital and the resultant reduced exposure to the risk of hospital-acquired infections. |
Our Corporate Strategy
Key elements of our strategy include:
| Ÿ | further develop SCY-078 to obtain regulatory approval in major commercial markets; |
| Ÿ | commercialize SCY-078 in the United States through a focused hospital-based sales force; |
| Ÿ | contract with commercial partners to develop and commercialize SCY-078 outside of the United States; and |
| Ÿ | leverage our strong scientific team and extensive in-house expertise in human and animal drug development to pursue the development of proprietary compounds. |
Overview of the Anti-Fungal Market
Background of Fungal Diseases
Candida and Aspergillus species are responsible for approximately 85% of all invasive fungal infections in the United States and Europe. Infections caused by Candida rank as the fourth most common hospital-acquired bloodstream infection in the United States. There are approximately 400,000 cases of invasive Candida infections annually worldwide. Invasive Candida infections result in a mortality rate ranging from 27% to 42% depending on the immune status of the patient. Globally, an estimated 150,000 patients develop confirmed invasive Aspergillus infections annually and over 50% of these patients die, even with treatment.
73
Hospital-acquired fungal infections due to Candida and Aspergillus species are becoming an increasing problem for the healthcare system. The increases in invasive fungal infections are due to the increased use of immune-suppressing chemotherapies and transplant drugs, and in-dwelling catheters, among other factors. Confirmed cases of invasive Candida infections rose in the United States by 52% between 2000 and 2005. In addition, the increase in use of broad spectrum antibiotics has been shown to contribute significantly to the risk of developing invasive fungal infections. Confirmed cases of invasive Aspergillus infections nearly doubled in the United States among patients receiving hematopoetic stem cell transplants between 2002 and 2005.
We believe confirmed cases of Candida blood infections account for only approximately one-quarter to one-third of Candida treatments. We further believe therapy prior to diagnosis, based on the presence of symptoms, represents a majority of the non-confirmed Candida treatments. This “empiric” therapy is clinically warranted because invasive Candida infections can be difficult to diagnose and the diagnosis is often available only after the patient has become too ill to recover. Initiation of therapy within the first twelve hours following suspicion of fungal infection reduces the risk of death by threefold. In addition, increased numbers of patients are undergoing procedures, such as chemotherapy and solid organ and stem cell transplants, that cause or result in immune-suppression and therefore put patients at high risk of invasive Candida infections. As a result, we believe anti-fungal therapy as preventative treatment accounts for the remaining Candida treatments.
Current therapeutic options
Invasive fungal infections are currently treated using three main classes of anti-fungal drugs that target fungal cell membranes or cell walls. Each of these anti-fungal drugs has its own limitations that reduces its clinical usefulness.
Azoles. Azoles, which block biosynthesis of a fungal cell membrane component, are the most frequently used class for treatment of invasive fungal infections and are available in IV and oral formulations. Azoles are used extensively for prevention and in unconfirmed cases. However, while azole-sensitive species have been well-treated, this has permitted azole-resistant infections, with species such as Candida glabrata, to become more prevalent. Further, cross resistance among the azoles exists, which means that once an azole has been tried and failed, another azole will likely not be effective. Despite these limitations, annual sales of azoles exceeded $2.1 billion in 2011. Voriconazole, the leading azole, generated revenues of $754 million in 2012.
Echinocandins. Echinocandins block biosynthesis of fungal cell walls by inhibiting a glucan synthase enzyme, an enzyme not found in human cells. The clinical success of echinocandins, particularly in azole resistant infections, combined with their good tolerability profile, has resulted in these compounds being increasingly used in the treatment of invasive Candida infections. However, echinocandins are only available in IV formulation. To allow for discharge from the hospital as quickly as possible, preferred medical practice is to transition eligible patients from IV to oral therapy. Without the availability of an oral echinocandin, physicians are forced to choose between administering oral azoles as a step down therapy and thereby risk re-emergence of an infection which may be azole resistant, or keeping the patient on an IV therapy, which may require continued hospitalization. Despite limitations as an IV-only therapy, annual sales of echinocandins were approximately $1.2 billion in 2011. Caspofungin, the leading echinocandin, generated revenues of $619 million in 2012.
Polyenes. Polyenes disrupt fungal cell membranes. The primary commercial polyene, amphotericin B, is used to treat a wide variety of fungi, including rare and difficult-to-treat species. However, polyenes have
serious side effects including acute, potentially fatal kidney and heart injury. As a result, polyenes are
74
typically used as a drug of last resort for treating invasive Candida and Aspergillus infections. Despite this toxicity, annual sales of lipid amphotericin B alone were approximately $450 million in 2012.
Anti-fungal Drug Resistance
Broad use of azole drugs has resulted in an increasing incidence of drug resistant Candida infections. At hospitals performing medically intensive procedures such as transplantation, rates of reduced azole susceptibility have reached 15-20%. We believe the rising level of azole resistance is driven by the emergence of resistance among previously susceptible species, such as Candida albicans, and the growing prominence of infections caused by species inherently resistant to azoles, such as Candida glabrata and Candida krusei. Declining azole efficacy in Candida infections has caused echinocandins to emerge as drugs of first choice for most patients with invasive Candida infections. However, a recent study reported echinocandin resistance for Candida glabrata at an incidence rate exceeding 10%. Of the echinocandin resistant strains, the majority are also resistant to azoles, making these strains multi-drug resistant.
Broad use of azole drugs has also fostered resistance in Aspergillus species. In a 2010 study, two U.S. laboratories reported resistance rates of approximately 50% in the Aspergillus fumigatus species, which accounts for the majority of Aspergillus fungal infections in the United States. These results were corroborated in another study, in which azole-resistant mutations were observed in approximately half of the Aspergillus samples evaluated from patients diagnosed with invasive Aspergillus lung infections.
Our Product Candidate: SCY-078
SCY-078 Overview
We discovered and developed SCY-078 through a research collaboration with Merck Sharp & Dohme Corp., or Merck, a subsidiary of Merck & Co., Inc., and in May 2013 acquired worldwide rights to SCY-078 in the field of human health. The compound is derived, by chemical modification, from a natural product that shows anti-fungal activity against Candida and Aspergillus through inhibition of glucan synthesis, like the echinocandin class. SCY-078 was shown to exhibit fungicidal activity against Candida albicans, the most common cause of invasive fungal infections among the Candida species, consistent with that of the echinocandins. In addition, SCY-078 has shown potent in vitro activity against approximately 800 laboratory and clinically important strains of Candida and Aspergillus, including strains that are resistant to azoles and echinocandins. The activity against echinocandin resistant strains suggests that SCY-078 represents a new class of anti-fungal agents that acts on a validated anti-fungal target in a manner distinct from the echinocandins.
In animal models of invasive fungal infections used to test other drugs that have proven to be effective in humans, SCY-078 was shown to be highly active against Candida and Aspergillus species. Further studies performed in these animal models allowed for the determination of the drug concentrations in blood required to achieve full anti-fungal effect. These correlations of drug exposure to drug activity, or PK/PD, have been used to identify the predicted human dose believed to be required to achieve adequate levels of anti-fungal activity.
In Phase 1 studies, SCY-078 has been shown to be sufficiently safe and well-tolerated in over 100 healthy human subjects at initial oral doses of up to 1800mg in one day and doses up to 800mg per day for 28 consecutive days to support progression into Phase 2 studies. Furthermore, oral dosing of the compound results in sustained blood concentrations in the range predicted from preclinical PK/PD studies to be required for adequate levels of anti-fungal activity. We plan to initiate a randomized Phase 2 study of the oral formulation of SCY-078 for invasive Candida infections in the first half of 2014. We are developing an IV formulation of SCY-078 and expect it will be available for Phase 2 trials in the first half of 2015.
75
In connection with our acquisition of the worldwide rights to SCY-078, Merck transferred to us responsibility for the investigational new drug application, or IND, for SCY-078, including all related technical documents, preclinical data, data from the seven Phase 1 trials conducted by Merck, and drug product and drug substance. The drug supplies included sufficient amounts of SCY-078 to complete the planned Phase 2 clinical trials for the oral formulation. Merck also transferred additional quantities of active pharmaceutical ingredient, which we believe will be sufficient to support development and manufacture of an IV formulation for clinical studies and provide material for additional toxicology studies.
The Generating Antibiotics Incentives Now Act, or GAIN Act, was enacted in July 2012 to encourage the development of novel anti-infective drugs in the face of increasing drug resistance. Before the passage of the GAIN Act, the FDA traditionally required sponsors of novel anti-fungal drugs to use non-life threatening fungal infections, such as esophageal Candida infections, for a proof-of-concept study in preparation for Phase 3 studies in invasive disease. This approach added time and cost to the process of developing novel drugs for invasive fungal infections. In order to encourage the development of treatments for serious or life-threatening infections, the GAIN Act required the FDA to review and ensure clear guidelines for clinical development of antibacterial and anti-fungal drugs. After receiving rights to SCY-078 in May 2013, in September 2013 we met with the FDA and were authorized by the FDA to proceed with smaller scale Phase 2 studies directly in patients with invasive Candida infections, our intended patient population, without first conducting studies of esophageal Candida infections. These changes, we believe, may significantly reduce the time and expense associated with progressing SCY-078 through Phase 2 and Phase 3 studies.
The FDA has designated the oral form of SCY-078 as a Qualified Infectious Disease Product, or QIDP, under the GAIN Act. The QIDP designation provides, among other benefits, increased access to the FDA during the development process as a fast track product, priority review once an NDA is submitted, and, if SCY-078 is approved for its proposed use and awarded five years of exclusivity as a new chemical entity, SCY-078 will be eligible for a ten-year period of data exclusivity, comprising five years of NCE exclusivity plus an additional five years as a designated QIDP. This exclusivity period should protect SCY-078 from being referenced in an abbreviated new drug application, or ANDA, in support of a generic drug, or a 505(b)(2) new drug application for a follow-on product until the expiration of the exclusivity period, which may be shortened by one year if an ANDA or 505(b)(2) applicant seeks to challenge any of the patents that claim SCY-078. We are submitting an additional application for the IV form of SCY-078 which we anticipate will be granted within the 60 day review period.
SCY-078 is protected by an issued composition of matter patent in the United States which provides exclusivity through 2030. We have licensed rights to develop and commercialize SCY-078 in the field of human health in Russia and certain smaller non-core markets to R-Pharm, CJSC, or R-Pharm, a leading supplier of hospital drugs in Russia, in exchange for an upfront payment, royalties, and their expertise and financial assistance in developing the compound.
SCY-078 Target Product Profile
We believe that there is significant commercial opportunity for a new anti-fungal drug that has potent activity against azole and echinocandin susceptible and resistant Candida and Aspergillus strains, available in both oral and IV formulations, and presents a favorable toxicity profile. SCY-078 has the potential to address all of these needs and could be used as follows:
Treatment of invasive Candida infections. If SCY-078 is proven safe and effective for the treatment of invasive Candida infections, we believe that it could overtake the echinocandins as the drug of choice for these infections because it will be available as both an IV and oral form. More than mere convenience, an orally effective anti-fungal would allow patients to be transitioned more easily from hospital-based care to outpatient care which would reduce, or eliminate, expensive hospital stays.
76
Treatment of infections with drug resistant Candida. SCY-078 has been shown to be effective preclinically against Candida species inherently resistant to azoles, such as Candida glabrata and Candida krusei, and against azole resistant strains of other species such as Candida albicans. In addition, SCY-078 has been shown to be effective preclinically against the majority of echinocandin-resistant Candida strains tested. SCY-078 could provide a first line treatment against invasive Candida infections known to be resistant to currently available azoles and echinocandins.
Treatment of invasive Aspergillus infections. If SCY-078 is proven safe and effective in treating invasive Aspergillus infections, we believe the drug would offer significant advantages over the current first line azole therapy of voriconazole due to the liver toxicity issues associated with the use of voriconazole. Furthermore, SCY-078 has been shown to be effective preclinically against all azole-resistant strains of Aspergillus tested. SCY-078 could provide a first line treatment against invasive Aspergillus infections known to be resistant to currently available azoles.
Prevention of Candida and Aspergillus infections. If proven to be safe and effective when used as a preventative treatment for Candida and Aspergillus infections, SCY-078 would offer advantages over current prophylactic drugs because of its activity against fungal strains that are resistant to azoles.
Preclinical Characterization of SCY-078
SCY-078 has broad anti-fungal activity based on a proven mechanism of action
SCY-078 is a potent inhibitor of the synthesis of the fungal cell wall polymer glucan, an essential component of Candida and Aspergillus species. Glucan synthesis inhibition is a clinically proven anti-fungal mechanism, as demonstrated by the echinocandin class of anti-fungal agents. Activity of SCY-078 observed against echinocandin-resistant strains suggests that SCY-078 acts in a manner distinct from the echinocandins.
SCY-078 is active in vitro against a broad spectrum of Candida and Aspergillus species
SCY-078 has been shown to have potent activity in vitro against over 600 strains from eleven Candida species and over 150 strains from four Aspergillus species. The charts below summarize the in vitro activity of SCY-078 against a collection of “wild-type” strains (i.e., those having no known drug resistance) of Candida and Aspergillus. Drug activity was measured as the minimum concentration of drug which inhibits replication of Candida or growth of Aspergillus by more than 50% relative to untreated cultures (MIC50 and MEC50, respectively). Each data point represents the average activity value for all strains tested at a single laboratory. Four laboratories were used for evaluation of Candida and three laboratories were used for evaluation of Aspergillus to confirm reproducibility of results among independent test sites. The potency of SCY-078 against these Candida and Aspergillus strains is comparable, within assay variability, to that of caspofungin, the current leading echinocandin.
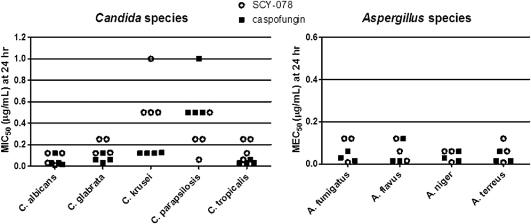
77
SCY-078 is active in vitro against azole-resistant Candida and Aspergillus strains
Overuse of azole drugs has allowed azole-resistant strains of Candida and Aspergillus to become increasingly prevalent, leading to treatment failures. Cross resistance among the azoles means that once an azole has been tried and failed, another azole will likely not be effective. SCY-078 was active against all azole-resistant Candida strains tested, with activity comparable to that observed against wild-type strains. As shown in the graph below, the in vitro activity of SCY-078 was comparable to that of the leading echinocandin against Candida albicans resistant to fluconazole, a leading azole.
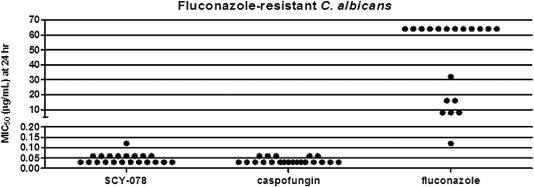
SCY-078 was also active against all azole-resistant Aspergillus strains tested, with the range of MEC50 values comparable to those observed against wild-type strains.
SCY-078 is active in vitro against a majority of echinocandin-resistant Candida species
Echinocandin resistance is also increasing, particularly among azole-resistant species such as Candida glabrata. As demonstrated in the chart below, SCY-078 retained more potent in vitro activity than did the leading echinocandin against a majority of echinocandin-resistant Candida glabrata strains tested. Similar results were observed for echinocandin-resistant strains of other Candida species. Thus, SCY-078 may offer a therapeutic option against multi-drug resistant strains such as those that have emerged in Candida glabrata.
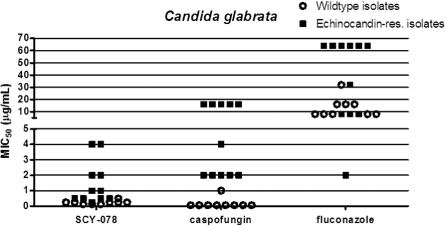
SCY-078 caused no major toxic effects in preclinical studies at therapeutic dose levels
The preclinical safety of SCY-078 has been evaluated in nine exploratory and two GLP, or Good Laboratory Practice, studies in rats, dogs, rabbits, and nonhuman primates. The longest duration of oral dosing was 28 days.
78
In these studies, at the highest tested doses, very slight to moderate toxicities were observed in two animal species. The two major organs impacted were the stomach (degeneration of the stomach lining) and the liver (single cell necrosis) and these effects were reversible upon cessation of dosing. The effect of degeneration of the stomach lining observed in preclinical toxicology studies was not reproduced in humans, even at exposure levels that exceeded those in the animal studies. In preliminary developmental and reproductive toxicity studies, SCY-078 did not cause any developmental toxicity in two animal species up to the maximum tolerated dose. In safety pharmacology studies, there were no clinically significant effects of SCY-078 on markers of cardiovascular, respiratory or central nervous system function.
Preclinical pharmacokinetic and drug metabolism properties of SCY-078 support effective oral administration and limited drug-drug interactions
SCY-078 has been evaluated broadly in preclinical pharmacokinetic and drug metabolism studies. SCY-078 was orally bioavailable in all four animal species studied, at levels that indicate the ability for effective oral dosing in the clinic.
Many patients with, or at risk of, invasive fungal infections are taking other medications, making it important to consider drug-drug interactions. The leading azoles have significant effects on the metabolism of many medications, which can lead to under-dosing or toxicity from co-administration of drugs. In contrast to most azoles, SCY-078 does not broadly inhibit drug metabolizing enzymes, and thus we anticipate that SCY-078 will have fewer drug-drug interactions.
In vivo animal studies predict that SCY-078 is effective against invasive fungal infections
Mouse models of Candida and Aspergillus infections have been predictive of clinical efficacy for all approved glucan synthesis inhibitors. SCY-078 was evaluated in multiple studies in Candida albicans-infected mice. In these studies, SCY-078 cured animals at doses which resulted in drug levels in the blood comparable to those that have been safely achieved in humans with other drugs. Similar results were observed in mice infected with other Candida species, including Candida glabrata.
The in vivo efficacy of SCY-078 was also evaluated against Aspergillus fumigatus in multiple studies. When infected with Aspergillus, mice with partially deficient immune defenses develop aggressive infections that generally result in death. However, SCY-078-treated mice exhibited dose-dependent increases in survival rates up to 90%, as measured in the first 21 days after infection.
In summary, SCY-078 demonstrated potent in vivo anti-fungal activity in all mouse models of Candida and Aspergillus infection studied, supporting our expectation of clinical efficacy for SCY-078.
Clinical Experience with SCY-078
To date, seven Phase 1 safety and pharmacokinetic studies have been completed using SCY-078. Four of the seven studies evaluated a single oral dose while three evaluated multiple oral doses of SCY-078.
SCY-078 consistently showed sufficient safety and tolerability in Phase 1 studies to support progression into Phase 2 studies
Over 100 healthy subjects have received at least one dose of SCY-078 in seven Phase 1 studies. SCY-078 was generally well tolerated at initial oral doses of up to 1800mg in one day and doses up to 800mg per day for 28 consecutive days. The majority of reported adverse events have been generally transient and primarily mild to moderate in intensity.
The preliminary safety and PK data from the completed Phase 1 studies are summarized in the following table:
79
| Design/Objective |
Clinical Endpoints |
Subject |
Dosing Regimen |
Results | ||||
| Phase 1, randomized, double-blind, placebo-controlled, single ascending-dose, safety, tolerability, and PK study | Safety and tolerability by physical examination, vital signs, ECGs and laboratory safety evaluations (hematology, chemistry, urinalysis), gastrin levels; PK data in fasted state and after high fat meal |
16 healthy males (18–45 years) | Panel A: 8 subjects: single doses 10, 40, 150, 600, and 1600mg SCY-078 (6 active / 2 placebo for each dose) Panel B: 8 subjects: single doses 20, 80, 300, and 800mg SCY-078 (6 active / 2 placebo for each dose) |
Safety: SCY-078 up to 1600mg was generally safe and well tolerated; no serious AE reported. PK parameters were approximately dose proportional for doses up to 1600mg Median Tmax 4 to 6 hours post dose (range 2-8 hr) Mean terminal half life ~ 20 hours Dosing with high fat meal increased AUC and Cmax by ~20%, within intersubject variability | ||||
| Phase 1, double-blind randomized, single dose study to evaluate the safety, tolerability, and PK in elderly subjects | Safety and tolerability by physical examination, vital signs, ECGs and laboratory safety evaluations (hematology, chemistry, urinalysis); PK data |
17 healthy males and females (65–85 years) | Panel A: 500 mg SCY-078/Placebo Panel B: Placebo/500 mg SCY-078 (6 active / 2 placebo for each panel) |
Safety: SCY-078 generally well tolerated. One non-drug-related SAE of metastatic carcinoid tumor was reported. Most common AEs were gastrointestinal disorders and nervous system disorders PK: Geometric mean ratios for AUC0-¥ in pooled elderly was approximately 5% higher than in young males with intersubject variability Median Tmax was ~50% earlier in pooled elderly as compared to young male subjects Mean T1/2 was approximately twice as long in pooled elderly as compared to young male subjects | ||||
| Phase 1, Open label biocomparison study of two formulations of SCY-078 and a pantoprazole interaction study with SCY-078 in healthy subjects | Safety, tolerability and PK of fit-for-purpose (FFP) drug filled capsules compared to FFP compressed tablets; impact of multiple doses of a proton pump inhibitor on single doses of SCY-078; impact of high fat meal on FFP compressed tablets | 16 healthy males (18–45 years) | Periods 1 and 2: Single doses of 500 mg SCY-078 (as five 100mg FFP dry filled capsules or two 250mg FFP compressed tablets) Period 3: Pantoprazole 40mg X 5 days and 500 mg SCY-078 (two 250mg FFP compressed tablets) Period 4: 500 mg SCY-078 (two 250mg FFP compressed tablets) administered after a high fat meal |
Safety: SCY-078 generally well tolerated. One SAE of elevated liver enzymes that led to discontinuation was reported. Most common AEs were gastrointestinal disorders PK: Geometric mean ratios for AUC0-¥ and Cmax were approximately 20% higher with compressed tablets as compared to dry filled capsules Geometric mean ratios AUC0-¥ were ~30% lower when SCY-078 was administered with as compared to without proton pump inhibitor Geometric mean ratios for AUC0-¥ and Cmax were approximately 45% higher with food than fasted | ||||
80
| Design/Objective |
Clinical Endpoints |
Subject |
Dosing Regimen |
Results | ||||
| Phase 1, randomized, double-blind, placebo-controlled, multiple ascending-dose safety, tolerability and PK study | Safety and tolerability by physical examination, vital signs, ECGs and laboratory safety evaluations (hematology, chemistry, urinalysis), gastrin levels and gastric histology; Plasma PK data and concentrations of intact drug in urine after multiple doses of SCY-078 |
32 healthy males (18–45 years) | 300, 600, and 800 mg SCY-078 or matching placebo once daily for 10 days, or 800 mg SCY-078 or matching placebo once daily for 28 days. (6 active /2 placebo in each panel) |
Safety: SCY-078 was generally safe and well tolerated. Most common AEs were headache, lack of energy, dizziness, nausea, vomiting and abdominal pain PK: True steady state geometric mean AUC 0-24 hr of at least 17µMŸhr was approached after 10 days of dosing at the 600mg dose Half life on day 10 was ~ 30 hrs Insignificant concentrations of SCY-078 were found in urine using an exploratory assay. Statistical analysis of trough concentrations indicated steady state not reached until after 2 weeks in many subjects Geometric mean (90%CI) accumulation ratios for AUC 0-24 hr and Cmax at steady state (day 26/day1) were 3.33 (2.58, 4.30) and 2.35 (1.85, 3.00) | ||||
| Phase 1, randomized, partially-blind, placebo-controlled study of multiple doses of ketoconazole on single dose PK of SCY-078 | Safety and tolerability of SCY-078 Single dose PK profile of SCY-078 after multiple doses of ketoconazole |
12 healthy males (18–45 years) | Period 1: 100 mg SCY-078 or matching placebo Period 2: Ketoconazole 400 mg once daily for 15 days starting on Day -1 with a single dose of 100 mg SCY-078 (or placebo) coadministered on Day 1. 12 Subjects (10 active / 2 placebo) |
Safety: SCY-078 was generally well tolerated when dosed alone or with ketoconazole. Most common AEs were headache and increased ALT/AST (N=2, SCY-078, 1 placebo) Geometric mean ratios for AUC0-¥ was ~ 5.7 fold higher when SCY-078 was administered with ketoconazole than when administered alone Terminal half life of SCY-078 with ketoconazole increased ~ 2 fold Cmax was increased ~ 2.5 fold in presence of ketoconazole | ||||
| Phase 1, randomized, double-blind, placebo controlled multiple dose study to assess the safety, tolerability, and PK of a loading dose of SCY-078 | Safety and tolerability of SCY-078; PK profile of SCY-078 after a loading dose on day 1 | 8 healthy males (18–45 years) | 1800 mg SCY-078 (or placebo) administered as 600 mg TID on Day 1, followed by 500 mg SCY-078 (or placebo) QD on Days 2-7. 8 Subjects (6 active / 2 placebo) |
Safety: SCY-078 was generally well tolerated. No SAEs or discontinuations. Most common AE diarrhea; 1 subject had elevated bilirubin PK: Geometric mean (90%CI) for AUC 0-24hr on day 1 with the loading dose was 20.8µMŸhr (15.8, 27.3) Day 3 and Day 7 geometric mean (90% CI) AUC0-24hr after SCY-078 500mg once daily were 20.8 (15.8, 27.4) and 16.0 (12.2, 21.0) respectively | ||||
81
| Design/Objective |
Clinical Endpoints |
Subject |
Dosing Regimen |
Results | ||||
| Phase 1, open-label, fixed-sequence, multiple-dose study investigating the effect of diltiazem on the PK and safety of SCY-078 in healthy subjects | Safety and tolerability of SCY-078; PK profile of SCY-078 after multiple doses of diltiazem | 16 males (20-45 years) |
Treatment A (Period 1), 200 mg SCY-078 q6h (total dose of 600 mg) on Day 1 and 100 mg SCY-078 QD Days 2 to 14. Treatment B (Period 2), 240 mg of diltiazem QD on Days -1 to 14, 200 mg of SCY-078 q6h (total dose of 600 mg) on Day 1, and 100 mg SY-078 QD Days 2 to 14. |
Safety: SCY-078 generally well tolerated. Most common AE was headache. No SAEs; 1 discontinuation due to first degree heart block (not drug related) PK: Geometric mean ratios for SCY-078 AUC0-24 hr was ~ 2.50 fold higher and for Cmax ~ 2 fold higher after 14 day co-administration than when dosed alone Median Tmax 5.00 hrs was unchanged | ||||
The most frequently reported adverse events have been gastrointestinal. In multiple dose studies, these included diarrhea, abdominal pain or discomfort, and vomiting. These gastrointestinal side effects were not considered serious in nature and only one subject discontinued dosing with SCY-078 due to gastrointestinal adverse events. In one study six subjects who received 800mg SCY-078 daily for 28 days underwent pre-treatment and end-of-treatment gastric endoscopy with biopsy, with no evidence of stomach lining degeneration or other significant clinical finding observed. No subjects in our Phase 1 studies were shown to have elevated serum gastrin levels.
One subject experienced significant liver function test increases after first dose and discontinued SCY-078 due to this serious adverse event, deemed by the investigator to be study drug related. However, markers of liver injury (ALT and AST) were already increasing prior to the subject receiving SCY-078 and pre-treatment levels of ALT had increased above the upper limit of normal. Other markers of liver injury remained within the normal range. ALT/AST levels decreased over the 48-hour period post-dose and this subject’s liver function tests returned to the normal range without intervention. This 27 year old man had no significant medical history and received 500mg of SCY-078. Evaluation revealed no clear etiology for the transaminase elevations. One other serious adverse event was reported: the subject was diagnosed with metastatic carcinoid tumor after one dose of SCY-078 and this was deemed not related to the study drug.
SCY-078 exhibits favorable pharmacokinetic properties in humans
As a result of seven Phase 1 studies of SCY-078, we believe that SCY-078 can be sufficiently well absorbed as an oral medication to achieve the drug levels necessary to be effective in treating patients . The half life of ~20 hours supports once daily dosing and a loading dose on day 1 should result in therapeutic concentrations being achieved on the first day of treatment. Drug exposure increased proportionally and in a predictable manner with doses up to the maximum dose tested (1600mg in single dose studies). There were no major differences in the pharmacokinetics or safety of SCY-078 in healthy elderly subjects relative to younger adults, an important consideration since many patients experiencing invasive fungal infections are elderly. Results from the two studies conducted to determine the potential for clinical drug-drug interactions confirmed that SCY-078 can be used in combinations with inhibitors of common drug metabolizing enzymes, with suitable dose adjustments. We therefore believe that SCY-078 will be orally bioavailable at therapeutically relevant dose levels which can be managed to avoid drug-drug interactions.
The drug interaction studies were performed with ketoconazole (strong inhibitor of CYP3A4) and diltiazem (moderate inhibitor of CYP3A4). Results of these studies indicate that a dose reduction of SCY-078
82
will be required with moderate CYP3A inhibitors and coadministration with strong inhibitors will not be recommended. A drug interaction study was also conducted with pantoprazole, a proton pump inhibitor. In this study, SCY-078 concentrations with pantoprazole were ~25% higher than SCY-078 alone; the results met the hypothesis that exposures of SCY-078 with or without a proton pump inhibitor were similar.
A biocomparison study was conducted between drug filled capsules that were used in early Phase 1 studies and compressed tablets which will be used in future studies. Compressed tablets had concentrations that were ~20-25% higher than capsules. The effect of a high fat meal on SCY-078 when dosed as compressed tablets indicated exposures that were ~50 to 60% higher than when administered in a fasted state.
Our clinical data, together with mouse efficacy data, support therapeutic activity for SCY-078
Correlations of circulating drug levels to drug efficacy in preclinical mouse infection models can be translated into human patients and are an established tool in the development of anti-fungal drugs. The efficacious drug levels determined for SCY-078 in the mouse models indicate that the levels achieved in the human Phase 1 clinical trials are predictive of efficacy in infected patients. Specifically, in human subjects who received SCY-078 as a loading oral dose of 600mg three times per day (1800mg/day) followed by a maintenance daily dose of 500mg, the circulating levels of SCY-078 exceeded those that cured the infection in the mouse models of invasive Candida infections. These results indicate that SCY-078 can be administered to patients with invasive Candida infections at doses that are predicted to be effective and generally well tolerated.
Future Clinical Development Plans for SCY-078
Based on results from studies to date, we believe that SCY-078 has the potential to offer a safe and effective new therapeutic option against fungal infections. The goal of the clinical development plan for SCY-078 is to provide sufficient safety and efficacy data for submission of an NDA.
We anticipate that our initial filing would seek an indication for oral and IV formulations of SCY-078 for the treatment of invasive Candida infections. We expect additional Phase 3 and Phase 4 studies to expand the list of indications to include treatment of invasive Aspergillus infections, and prevention of invasive fungal infections.
SCY-078 Phase 2 studies
In consultation with regulatory agencies, we plan to pursue the following Phase 2 studies to evaluate the safety and efficacy of SCY-078 in subjects with invasive fungal infections caused by Candida.
SCY-078 as an Oral Step-Down in the Treatment of Invasive Candida Infections: SCY-078 will be used as an oral step-down agent following initial therapy with a currently available IV echinocandin in patients with invasive Candida infections. The open label study will recruit approximately 90 patients and will include: patients infected with Candida glabrata and Candida krusei, species of Candida which are resistant to azoles; patients who were previously treated with azole therapy for a prior fungal infection; and treatment naïve patients. This will be a three arm study comparing step-down oral therapy with two doses of SCY-078 to current standard of care. All subjects will initiate therapy with an IV echinocandin for three to five days. Patients in arm one will switch to oral SCY-078 dosed at 500mg three times a day on day one followed by once daily dosing of SCY-078 500mg for up to 27 days. Patients in arm two will switch to oral SCY-078 dosed at 750mg three times a day on day one followed by once daily dosing of SCY-078 750mg for up to 27 days. Patients in arm three will receive standard of care. Current standard of care calls for a switch to oral therapy with an azole for up to 28 days, unless the patient is infected with azole resistant or
83
refractory Candida in which case the patient will be maintained on IV echinocandin, which treatment is also for up to 28 days. We expect to initiate this study in the first half of 2014. Due to the open-label nature of the studies, we expect to be able to report preliminary results following enrollment of 50% of study subjects, which we expect to achieve in the first quarter of 2015.
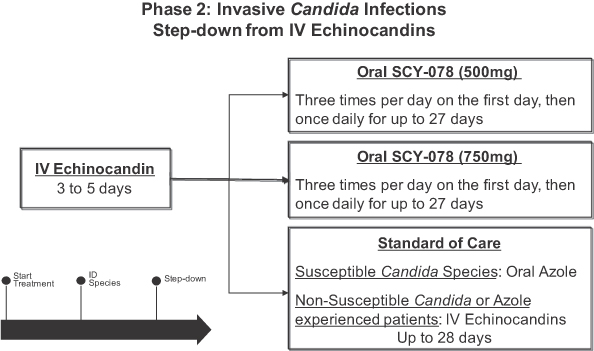
SCY-078 (IV and Oral) for the Treatment of Invasive Candida Infections: We are developing an IV formulation and expect it will be available for Phase 2 studies in the first half of 2015. A second Phase 2 study will evaluate the safety and efficacy of SCY-078 in the treatment of invasive Candida infections. Treatment-experienced subjects, prior treatment with azoles and/or echinocandins, will be enrolled and treated with IV SCY-078, with an option of being switched to oral therapy with SCY-078.
SCY-078 Phase 3 study
As noted above, we are planning to seek an initial indication for SCY-078 as an oral/IV drug for the treatment of invasive Candida infections. We plan to conduct a Phase 3 study in subjects with invasive Candida infections including those with previous experience with azoles and/or echinocandins.
Acquisition of SCY-078 from Merck
In May 2013 Merck transferred to us all development and commercialization rights for SCY-078 (also known as MK-3118). This decision was made following a review and prioritization of Merck’s infectious disease portfolio. Under the terms of the agreement, we have received all human health rights to SCY-078, including all related technical documents, preclinical data, data from the seven Phase 1 trials conducted by Merck, and drug product and drug substance. Merck also transferred additional quantities of active pharmaceutical ingredient, which we believe will be sufficient to support development and manufacture of an IV formulation for clinical studies and provide material for additional toxicology studies. The agreement continues until expiration of all royalty obligations, at which point our license will become a fully paid-up,
84
perpetual license. The agreement may be terminated if either party is in material breach and fails to remedy the breach after receiving written notice. In 2014, Merck assigned the patents to us related to SCY-078 that it had exclusively licensed to us. Merck is eligible to receive milestones upon initiation of Phase 2 and 3 clinical studies, NDA filing and marketing approvals in each of the United States, major European markets and Japan that could total up to $19 million. In addition, Merck will receive tiered royalties based on worldwide sales of SCY-078. The aggregate royalties are mid- to high- single digit percentages of net sales.
Commercialization, Marketing and Sales of SCY-078
Given our stage of development, we have not yet established a commercial organization or distribution capabilities.
We expect that prescribing physicians for the treatment of invasive fungal infections will be located at major medical centers, where physicians specializing in critical care, infectious disease specialists, and physicians treating immune-compromised or immune-suppressed patients, such as oncologists and those performing solid organ transplants and stem cell transplants, are likely to be found.
We intend to form our own focused hospital-based sales and marketing force to target physicians in the United States. Outside of the United States, subject to obtaining necessary marketing approvals, we likely will seek to commercialize SCY-078 through distribution or other collaboration arrangements. We have already entered into an agreement pursuant to which we outlicensed to R-Pharm rights to develop and commercialize SCY-078 in the field of human health in Russia and certain smaller non-core markets.
Competition for SCY-078
Our competitors include large pharmaceutical and biotechnology companies, and specialty pharmaceutical and generic drug companies. The three leading branded anti-fungal drugs represent one from each main class; V-fend® (voriconazole), an azole marketed by Pfizer ($754 million in 2012); Cancidas® (caspofungin), an echinocandin marketed by Merck ($619 million in 2012); and AmBisome® (liposomal amphotericerin B), a polyene sold by Gilead in Europe, by Astellas in the United States and by Dainippon-Sumitomo in Japan ($450 million in 2012). Pfizer also markets the echinocandin Eraxis® (anidulafungin), Merck also markets the azole Noxafil® (posaconazole), and Astellas also markets the echinocandin Mycamine® (micafungin). Pfizer, Merck and Astellas are all large pharmaceutical companies with significant experience and financial resources in the marketing and sale of specialty pharmaceuticals. Various other producers market and sell generic oral voriconazole, fluconazole and itraconazole. Further, we expect that product candidates currently in late stage development, or that could enter late stage clinical development in the near future, may represent significant competition, if approved. These include the azole isavuconazole (under development by Basilea, with marketing rights to Astellas), VT-1161 being developed by Viamet, and MGCD290 being developed by Methylgene. These companies may have significantly greater resources than we have.
The key competitive factors affecting the success of SCY-078, if approved, are likely to be its efficacy, safety, convenience, price, use in out-patient settings, the level of generic competition and the availability of reimbursement from government and other third-party payors. If approved, we believe that SCY-078’s features, including its oral dosing and efficacy against resistant strains, will differentiate it from these competing products. We believe that SCY-078 will compete favorably against competing products in efficacy, safety, convenience and use in out-patient settings, allowing us to price SCY-078 at a premium to generics and other competing products.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain
85
FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours. In addition, our ability to compete may be affected because in many cases insurers or other third-party payors seek to encourage the use of generic products. In the azole class, fluconazole, itraconazole, and oral voriconazole are generic. There is currently no generic echinocandin, but caspofungin, the largest selling echinocandin, is expected to become available on a generic basis over the coming years and perhaps prior to the launch of SCY-078. If approved, we believe SCY-078 will be capable of delivering value supportive of premium pricing over competitive generic products.
Manufacturing and Supply of SCY-078
We have an in-house facility capable of supplying kilogram quantities of drug substance, and we can develop analytical procedures to support the preparation of clinical batches. However, we do not own or operate and do not expect to own or operate facilities for manufacturing, storage and distribution, or testing of drug substance or drug product for late stage clinical trials or commercial manufacture. In the past, we have relied on third-party contract manufacturers for large scale synthesis of our clinical compounds and manufacture of drug product. We expect to continue to rely on these manufacturers to supply SCY-078 for planned clinical trials and commercial sale.
SCY-078 is a semi-synthetic natural product. Thus, the manufacturing process for SCY-078 involves fermentation and synthetic chemical steps. The process begins with fermentation to produce the natural product enfumafungin, which has been conducted by a third-party vendor on a scale sufficient to provide greater than 60kg of this starting material. Enfumafungin is then converted to SCY-078 in a series of chemical steps that proceed efficiently with an average yield of almost 90%. Approximately 20kg of drug substance has been manufactured. The overall process does not require any specialized equipment and uses readily sourced intermediates. At commercial launch, we expect cost of goods for SCY-078 to be similar to that of other small molecule drugs. We are negotiating agreements with large scale suppliers to produce both drug product and drug substance for planned clinical trials. In the future, we plan to validate the process with selected vendors and secondary suppliers to establish a secure supply chain.
We expect the tablets currently on hand to be sufficient to complete our Phase 2 trials. They have shown good stability for one and a half years at four degrees centigrade storage condition. An IV formulation is under development, and we expect it to be completed by the second half of 2014.
A drug manufacturing program subject to extensive governmental regulations requires robust quality assurance systems and experienced personnel with the relevant technical and regulatory expertise as well as strong project management skills. We have a team that we believe is capable of managing these activities, and it has successfully supported our clinical drug for HCV, SCY-635, as well as numerous such programs for clients in our contract business. Our internal facilities have been FDA audited on two separate occasions with no notice of non-compliance.
Our Cyclophilin Inhibitor Platform
We have developed a proprietary platform for cyclophilin inhibitors. Cyclophilins are a family of enzymes found in all mammalian cells which play a key role in a number of important cellular functions. Inhibiting cyclophilins show promise as treatments for a range of diseases. To date, our cyclophilin inhibitor platform has produced two clinical stage compounds, described below.
SCY-635 is a novel, orally available cyclophilin inhibitor that has demonstrated clinical activity against Hepatitis C Virus (HCV) as a single agent and when dosed in combination with pegylated interferon and ribavirin. In these clinical studies, SCY-635 modified patients’ immune responses to HCV. These observations implicate cyclophilins in viral evasion of immune responses. We are further exploring this
86
mechanism in other viruses such as hepatitis B virus (HBV). HCV and HBV are two of the most widespread global infections, with more than 170 million and 240 million chronic carriers respectively, and are leading causes of liver cirrhosis, liver cancer and liver transplantation.
SCY-641 is a novel cyclophilin inhibitor with activity similar to cyclosporine, the active ingredient in Restasis® and Optimmune®, drugs currently approved for dry eye disease in humans and dogs, respectively. The global human dry eye syndrome therapeutics market was valued at $1.8 billion in 2010 and the market value is expected to grow to $2.8 billion in 2017. Sales of Restasis® in 2012 were $792 million. SCY-641 has significantly improved water solubility compared to cyclosporine which we believe will lead to improved tolerability and ease of use for treatment of dry eye disease, i.e., does not sting when applied and only requires dosing one to two times per day. In August 2012, we licensed worldwide animal health rights for SCY-641 to Dechra Ltd., while retaining rights for human health indications. We intend to identify a development and commercial partner for the human health uses of SCY-641.
We have a library of more than 1,000 other cyclophilin inhibitor compounds that could be effective against a wide variety of human and animal diseases. We plan to enter into corporate partnerships to use our cyclophilin inhibitor platform to discover and develop new drug candidates for unmet needs in human and animal health.
Our Contract Research and Development Services
As a spinout from Aventis in 2000, we began as a chemistry and animal health services company, providing contract research services to third parties. Through this business, we built significant expertise in parasitic infections and drug discovery. Since our formation, we have expanded our animal health capabilities and have discovered a number of proprietary compounds.
The market for parasiticides was estimated to be more than $5.5 billion globally in 2011. We have more than 30 unique, broad spectrum screens, and proprietary protocols and algorithms, deemed to be trade secrets. Our antiparasitic drug discovery platform has enabled us to discover drugs for our partners and has traditionally produced substantially all of our revenues.
In partnership with Merial, the animal health division of Sanofi, we have discovered two new drug candidates to treat parasitic infections. In addition, in a collaboration sponsored by the Bill & Melinda Gates Foundation, we discovered a drug that is now in Phase 1 studies for the treatment of “sleeping sickness,” a fatal disease transmitted to humans by biting flies in Sub-Saharan Africa. We have also leveraged our expertise and our cyclophilin inhibitor platform to discover SCY-641, a compound licensed to Dechra Ltd. in 2012 for clinical development for the treatment of dog dry eye.
We intend to continue to grow our contract research and development services and to leverage our in-house expertise for the discovery of additional proprietary compounds.
Collaborations and Licensing Agreements
We have a number of licensing and collaboration agreements with partners in human and animal health, including the following:
Merck
We have a termination and license agreement with Merck, as described under “Acquisition of SCY-078 from Merck” above.
87
Merial
Merial, a wholly owned subsidiary of Sanofi, is one of the largest animal health businesses in the world and has been our major partner in animal health since 2003. We signed a new agreement with Merial effective January 2012 under which we provide contract research and development services in the field of animal health. In contrast to our earlier agreement with Merial, this is a non-exclusive arrangement in the animal health field and is on a fee-for-service basis, meaning we will not receive any contingent payments based on the progression to development and commercialization of any compounds arising from this agreement. The term of this agreement is three years ending on December 31, 2014. Either party may terminate the agreement in the event of breach of material obligation by the other party if such breach is not remedied after written notice from the non-breaching party. Either party may terminate this agreement if the other party makes an assignment for the benefit of creditors, becomes subject to bankruptcy proceedings, subject to appointment of a receiver, or admits inability to pay its debts. If Merial believes in good faith that we acted in any way that may subject Merial to liability under anti-corruption laws, Merial shall have the unilateral right to terminate this agreement. At termination or expiration of the agreement for any reason, upon Merial’s request, we must transfer all agreement intellectual property to Merial. Merial accounted for 41% and 44% of our revenues in the nine months ended September 30, 2013, and the year ended December 31, 2012, respectively. No other customer accounted for 10% or more of our revenues during these time periods.
R-Pharm
In August 2013 we entered into an agreement with R-Pharm, a leading supplier of hospital drugs in Russia, granting them exclusive rights to develop and commercialize SCY-078 in the field of human health in Russia, Turkey, and certain Balkan, Central Asian, Middle Eastern and Northern African countries. We retained the right to commercialize SCY-078 in the Americas, Europe, and Asia. We received an upfront payment, are entitled to receive payments on development milestones, commercialization milestones based upon the cumulative net sales of the product, and generally low double digit percentage royalties on SCY-078 sales. This agreement expires upon R-Pharm’s last royalty payment, which is the later of twelve years from the first registration of the product in the countries where R-Pharm’s license rights exist under this agreement, or the last to expire of the patents in such countries. Either party may terminate this agreement if the other party breaches, and fails to remedy the breach after receiving notice from the non-breaching party. We have the ability to terminate this agreement if we determine that R-Pharm fails to make reasonable progress in the development and commercialization of SCY-078. If we give R-Pharm notice of failure to make reasonable progress, R-Pharm will have the opportunity to correct the deficiencies.
Dechra
In August 2012 we signed an agreement with Dechra Ltd., a UK listed international veterinary pharmaceutical business, granting Dechra rights to SCY-641 for use in the field of animal health, including the treatment of canine keratoconjunctivitis sicca, or dry eye in dogs. Dechra was granted worldwide animal health rights and is responsible for the remaining clinical development and commercialization of SCY-641 in the animal health field. We retained the human health rights to the compound, including the right to use preclinical data generated by Dechra to support further human clinical development. Under the agreement, Dechra must use reasonable efforts to commercialize SCY-641. We received an upfront fee and are eligible to receive further payments based on development milestones, as well as low to high double-digit royalties on total net sales of product sales. Dechra’s obligations to pay royalties shall continue, on a product-by-product and country by country basis, until there are no valid claims underlying the product in such country or a specified period of time after the first commercial sale in such country. This agreement expires when
88
Dechra has completed all royalty payment obligations. If either party is in breach, and the breach continues after notice given by the non-breaching party, the non-breaching party may terminate the agreement. If we terminate the agreement because Dechra is in breach, Dechra must return all information required to be returned under the license agreement, free of charge, to us. If Dechra reasonably believes it is impossible to carry out further development or marketing of animal health products, Dechra may terminate this agreement at anytime by giving us at least six months prior written notice. In November 2013, we amended this license agreement with Dechra in which we agreed to perform certain services for Dechra.
Aventis
In May, 2005, we entered into a license agreement with Aventis Pharma S.A., a leading global healthcare company, pursuant to which Aventis granted us a world-wide license (with a right to sub-license) to certain of Aventis’s know-how, compounds and patents concerning cyclosporine derivatives exclusively in the field of treatment and prevention of HIV/AIDS and non-exclusively in all fields outside the treatment and prevention of HIV/AIDS. Under the terms of the agreement, we are obligated to maintain reasonable efforts to develop and commercialize a marketable product containing the subject compound and Aventis is responsible for maintaining and protecting the underlying patent rights. The agreement expires on a country by country basis at the end of the underlying intellectual property claims. We may terminate the agreement at any time, without cause, by giving Aventis 90 days notice. Aventis may terminate this agreement only if we commit a serious breach and fail to remedy the breach within 90 days of notice. Upon expiration of the agreement, we will have a fully paid-up, royalty free, world-wide, exclusive license in the field of treatment and prevention of HIV/AIDS and a non-exclusive license outside this field. We are obligated to pay Aventis up to an aggregate of $1.35 million in payments upon the achievement of certain milestones. In addition, on an annual basis, we will be obligated to pay a single digit percentage royalty on direct sales by us of all products developed under the agreement and we will pay a low single digit percentage of royalty on any sales by a sub-licensee of all products developed under the agreement.
C-Chem
In June, 2005, we entered into an assignment agreement with C-Chem AG pursuant to which C-Chem assigned certain inventions, patents and know-how concerning cyclosporine derivatives for us to research, develop, manufacture and commercialize a product. Under the agreement, C-Chem has assigned to us all rights, title and interest in the subject patents as well as assigned all rights, title and interest to certain know-how with exclusive right to use and disclose the know-how for any purpose. Under the agreement, we must exercise reasonable commercial efforts to develop and commercialize a product using the licensed intellectual property and we are responsible for maintaining the licensed patents until the end of their lifetime. The agreement expires when no valid claim remains with respect to the underlying patents. C-Chem may terminate the agreement if an order by a court is made appointing a custodian, receiver, liquidator, assignee or trustee for us or if a court orders the winding up or liquidation of our affairs. We can terminate the agreement at any time by thirty (30) days written notice to C-Chem. If either party breaches any term or condition of the agreement, then the non-breaching party can terminate the agreement if notice is given to the breaching party and the breach is not remedied in sixty (60) days. Upon expiration of the agreement, we will have a fully paid-up, royalty free, world-wide exclusive license, and the right to grant sub-licenses, under the know-how and ancillary rights to commercialize and supply products. If the agreement is terminated by either party, we are obligated to reassign the patents, the know-how and the ancillary rights to C-Chem, return any intellectual property to C-Chem, and cease all activities which would require a license under the subject patents. We are obligated to pay C-Chem up to $0.95 million in payments upon the achievement of certain milestones. In addition, we will be obligated to pay a low single digit percentage royalty on direct sales by us of all products developed under the agreement and we will pay less than a 1% royalty on any sales by a licensee of all products developed under the agreement.
89
Elanco Animal Health
In December, 2013, we entered into a license, development, and commercialization agreement with Elanco Animal Health, the animal health division of Eli Lilly Company, an American global pharmaceutical company, pursuant to which we will perform research services and grant to Elanco a world-wide license (with a right to sub-license) to certain of our know-how, compounds, and patents exclusively for applications and uses of parasiticides for animals (companion or food), animal products, animal feed, human food, or the food chain. Under the terms of the agreement, both parties must use reasonable commercial efforts to collaboratively research and commercialize products. After the completion of the first half of the research phase, either party may terminate the research component of the agreement upon advance notice if the research is not progressing to the satisfaction of either party. The term of the agreement will survive until the expiration of the last remaining royalty term, provided, however, that Elanco may terminate the agreement upon advance written notice to us any time after termination or expiration of the research services term. In the event Elanco terminates the agreement, Elanco will grant us a fully paid-up, royalty free, world-wide non-exclusive license in the field with respect to any compound or product developed for Elanco under the agreement. Either party may terminate the agreement in an event of default of the other party, which includes a material breach of the agreement, failure on the part of Elanco to make any payments due, or the bankruptcy, insolvency or dissolution of either party. Elanco will pay us annually in the low millions for performing research services during the research services term. In addition, upon the achievement of certain milestones with respect to each compound developed under the agreement, we may be entitled to receive additional payments. We will also be entitled to receive quarterly royalty payments in the low to mid single digit on the net sales of each product developed and commercialized under the agreement.
Government Regulation and Product Approval
Government regulation
Government authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, including any manufacturing changes, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, import and export of pharmaceutical products such as those we are developing. The processes for obtaining regulatory approvals in the United States and in foreign countries, along with subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources.
U.S. drug approval process
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending NDAs, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters, product recall requests, product seizures, total or partial suspension of production or distribution, injunctions, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
90
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
| Ÿ | completion of preclinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s good laboratory practice, or GLP, regulations; |
| Ÿ | submission to the FDA of an investigational new drug application, or IND, which must become effective before human clinical trials may begin; |
| Ÿ | approval by an independent institutional review board, or IRB, at each clinical site before each trial may be initiated; |
| Ÿ | performance of adequate and well-controlled human clinical trials in accordance with good clinical practices, or GCP, to establish the safety and efficacy of the proposed drug for each indication; |
| Ÿ | submission to the FDA of an NDA; |
| Ÿ | satisfactory completion of an FDA advisory committee review, if applicable; |
| Ÿ | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP, and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| Ÿ | FDA review and approval of the NDA. |
Preclinical studies
Preclinical studies include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies to assess potential safety and efficacy. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data and any available clinical data or literature, among other things, to the FDA as part of an IND. Some preclinical testing may continue even after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to one or more proposed clinical trials and places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
Clinical trials
Clinical trials involve the administration of the investigational new drug to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include the requirement that all research subjects provide their informed consent in writing for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, an IRB at each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution. Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health, or NIH, for public dissemination on their ClinicalTrials.gov website.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
Phase 1: The drug is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness.
91
Phase 2: The drug is administered to a limited patient population with the target disease to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
Phase 3: The drug is administered to an expanded patient population with the target disease, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product.
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
In some cases, the FDA may condition approval of an NDA for a product candidate on the sponsor’s agreement to conduct additional clinical trials to further assess the drug’s safety and effectiveness after NDA approval. Such post-approval trials are typically referred to as Phase 4 studies.
In some circumstances, the FDA may also order a sponsor to conduct post-marketing clinical trials after approval of the product, if new safety information arises raising questions about the drug’s risk-benefit profile. Those clinical trials are typically referred to as Post-Marketing Requirements, or PMRs.
Marketing approval
Assuming successful completion of the required clinical testing, the results of the preclinical and clinical studies, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA requesting approval to market the product for one or more indications. In most cases, the submission of an NDA is subject to a substantial application user fee. Under Prescription Drug User Fee Act guidelines that are currently in effect, the FDA has a goal of twelve months from the date of the receipt of a standard non-priority NDA to review and act on the submission for a drug considered to be a new molecular entity, or eight months for a priority NDA for such drug.
In addition, under the Pediatric Research Equity Act of 2003, an NDA or supplement to an NDA must contain data that are adequate to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements.
The FDA also may require submission of a risk evaluation and mitigation strategy, or REMS, plan to mitigate any identified or suspected serious risks. The REMS could include medication guides, physician communication plans, assessment plans, and elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools.
The FDA conducts a preliminary review of all NDAs within the first 60 days after submission, before accepting them for filing, to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject
92
to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA reviews an NDA to determine, among other things, whether the drug is safe and effective and the facility in which it is manufactured, processed, packaged or held meets standards designed to assure the product’s continued safety, quality and purity. The FDA is required to refer an application for a novel drug to an advisory committee or explain why such referral was not made. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP.
The testing and approval process requires substantial time, effort and financial resources, and each may take several years to complete. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all.
If the FDA’s evaluation of the NDA and inspection of the manufacturing facilities are favorable, the FDA may issue an approval letter, or, in some cases, a complete response letter. A complete response letter generally contains a statement of specific conditions that must be met to secure final approval of the NDA and may require additional clinical or preclinical testing for the FDA to reconsider the application. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications.
Even if the FDA approves a product, it may limit the approved indications for use for the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess a drug’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-marketing studies or surveillance programs. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes, and additional labeling claims, are subject to further testing requirements and FDA review and approval.
GAIN Act
The FDA has various programs, including fast track designation and priority review, that are intended to expedite or simplify the process for the development and FDA review of drugs that meet certain qualifications. The purpose of these programs is to provide important new drugs to patients earlier than under standard FDA review procedures.
The GAIN Act is intended to encourage development of new antibacterial and anti-fungal drugs for the treatment of serious or life-threatening infections by providing certain benefits to sponsors, including extended exclusivity periods, fast track and priority review. To be eligible for these benefits a product in development must seek and be awarded designation as a QIDP.
93
To qualify as a QIDP according to the criteria established in the GAIN Act a product must be an antibacterial or anti-fungal drug for human use intended to treat serious or life-threatening infections, including, those:
| (1) | caused by an anti-fungal resistant pathogen, including novel or emerging infectious pathogens; or |
| (2) | qualifying pathogens listed by the FDA in accordance with the GAIN Act. |
In January 2014 the FDA designated the oral form of SCY-078 as a QIDP. We are submitting an additional QIDP application for the IV form of SCY-078 which we anticipate will be granted within the 60 day review period.
Post-approval requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to extensive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
The FDA may impose a number of post-approval requirements as a condition of approval of an NDA. For example, the FDA may require post-marketing testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| Ÿ | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| Ÿ | warning letters or holds on post-approval clinical trials; |
| Ÿ | refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product license approvals; |
| Ÿ | product seizure or detention, or refusal to permit the import or export of products; or |
| Ÿ | injunctions or the imposition of civil or criminal penalties. |
94
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution of drugs and drug samples at the federal level, and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Exclusivity and approval of competing products
Hatch-Waxman exclusivity
Market and data exclusivity provisions under the FDCA can delay the submission or the approval of certain applications for competing products. The FDCA provides a five-year period of non-patent data exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. As an alternate path to FDA approval for modifications to drug products previously approved by the FDA, or new indications for use of previously approved drug products, an applicant may file an NDA under Section 505(b)(2) of the FDCA. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The FDCA permits the applicant to rely upon certain preclinical or clinical studies conducted for an approved product. The FDA typically requires companies to perform additional, sometimes extensive, clinical studies and analyses to support the change from the approved product. The FDA may then approve the new product candidate for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant. During the exclusivity period for a new chemical entity, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company that references the previously approved drug. However, an ANDA or 505(b)(2) NDA may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA, or supplement to an existing NDA or 505(b)(2) NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant, are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages, strengths or dosage forms of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and, as a general matter, does not prohibit the FDA from approving ANDAs or 505(b)(2) NDAs for generic versions of the original, unmodified drug product. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Pediatric exclusivity
Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity, including the non-patent exclusivity periods described above. This six-
95
month exclusivity may be granted based on the voluntary completion of a pediatric study or studies in accordance with an FDA-issued “Written Request” for such a study or studies.
Qualified Infectious Disease Product exclusivity
We received QIDP designation for the oral form of SCY-078 and we are submitting an additional QIDP application for the IV form of SCY-078 which we anticipate will be granted within the 60 day review period. If the NDA to be submitted for SCY-078 is approved by the FDA, the FDA will extend by an additional five years any non-patent marketing exclusivity period awarded, such as a five-year exclusivity period awarded for a new chemical entity. This extension is in addition to any pediatric exclusivity extension awarded, and the extension will be awarded only to a drug first approved on or after the date of enactment, July 9, 2012. Eligibility for the extension will be denied if the product is approved for uses that would not meet the definition of a QIDP.
Foreign regulation
To market any product outside of the United States, we would need to comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we would need to obtain the necessary approvals by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others.
Pharmaceutical coverage, pricing and reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we may obtain regulatory approval. Sales of any of our product candidates which may be ultimately approved, including SCY-078, will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, including government health programs such as Medicare and Medicaid, and commercial health insurers. The process for determining whether a payor will provide coverage for a drug product is separate from the process for determining the reimbursement rate for the drug product once coverage is approved. Third-party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the approved drugs for a particular indication or may apply utilization management requirements such as prior authorization to restrict access to certain approved drugs for a particular indication.
To secure coverage and reimbursement for any product that might be approved by the FDA for sale, we may need to conduct expensive pharmacoeconomic studies to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. Our product candidates may not be considered medically necessary or cost-effective by government or private third-party payor decision makers. A payor’s decision to provide coverage for a drug product does not mean that the product will be adequately reimbursed. Third-party reimbursement may not be sufficient to enable us to realize an appropriate return on our investment in product development.
The containment of healthcare costs has become a priority of federal, state and foreign governments, and the prices of drugs have been a focus in this effort. Third-party payors are increasingly challenging the prices of medical products and corresponding services and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. If these third-party
96
payors do not consider products to be cost-effective compared to other available therapies, they may not provide coverage for our products after approval as a benefit under their health insurance plans or, if they do, the reimbursement rates may not be adequate to allow recovery of product development and production costs. In addition, and to be considered for coverage and reimbursement, all third-party payors in the United States require that healthcare providers use unique codes to identify the product and service rendered when billing for such products and services. Codes unique to a pharmaceutical product for use in a physician’s office, such as our lead product candidate, are only available after a twelve-month coding application and review process by the Centers for Medicare and Medicaid Services, or CMS, which commences in January of each year post FDA approval of the product. Codes for use in hospital outpatient departments may be created mid-year, but there may be delay between launch and issuance of a code. In the absence of a unique code for a pharmaceutical product post commercial launch, and in the interim, it is standard practice for healthcare providers in the United States to use a temporary code when billing third-party payors to describe the pharmaceutical product rendered.
The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs to limit the growth of government-paid health care costs, including price controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. Adoption of such controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals such as the drug product candidates that we are developing and could adversely affect our net revenue and results.
Pricing and reimbursement requirements vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies. For example, the European Union provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a drug product or it may instead adopt a system of direct or indirect controls on our profitability in placing the drug product on the market. Other member states allow companies to fix their own prices for drug products, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. There can be no assurance that any country that has price controls or reimbursement limitations for drug products will allow favorable reimbursement and pricing arrangements for any of our products.
The marketability and adoption of any products for which we receive regulatory approval for commercial sale may suffer if the government and private third-party payors fail to provide adequate coverage and reimbursement. In addition, emphasis on managed care in the United States has increased and we expect will continue to increase the pressure on drug pricing. Coverage policies, third-party payment rates and drug pricing regulation may change at any time. In particular, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010, together the Affordable Care Act, was adopted in the United States. This law substantially changes the way healthcare is financed by both governmental and private insurers, and significantly impacts the pharmaceutical industry. Changes that may affect our business if we or our partners commercialize our products in the future include those governing enrollment in federal healthcare programs, reimbursement changes, rules regarding prescription drug benefits under the health insurance exchanges, and fraud and abuse and enforcement. In addition, continued implementation of the Affordable Care Act may result in the expansion of new programs such as Medicare payment for performance
97
initiatives, and may impact existing government healthcare programs, such as by improving the physician quality reporting system and feedback program.
Additional provisions of the Affordable Care Act may negatively affect our revenues from products that we commercialize in the future. For example, as part of the Affordable Care Act’s provisions closing a coverage gap that currently exists in the Medicare Part D prescription drug program, manufacturers of branded prescription drugs are required to provide a 50% discount on branded prescription drugs dispensed to beneficiaries within this coverage gap. Medicare Part D is a prescription drug benefit available to all Medicare beneficiaries. It is a voluntary benefit that is implemented through private plans under contractual arrangements with the federal government. Similar to pharmaceutical coverage through private health insurance, Part D plans negotiate discounts from drug manufacturers and pass on some of those savings to Medicare beneficiaries. Effective March 23, 2010, rebates are also due on the drug utilization of Medicaid managed care organizations. With regard to the amount of the rebates owed, the Affordable Care Act increased the minimum Medicaid rebate for all drugs; changed the calculation of the rebate for certain innovator products that qualify as line extensions of existing drugs; and capped the total rebate amount for innovator drugs at 100% of the average manufacturer price, or AMP. In addition, the Affordable Care Act and subsequent legislation changed the definition of AMP. Finally, the Affordable Care Act requires pharmaceutical manufacturers of branded prescription drugs to pay a new branded prescription drug fee to the federal government beginning in 2011.
Even if favorable coverage and adequate payment status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and payment rates may be implemented in the future.
Healthcare law and regulation
Healthcare providers, physicians and third-party payors often play a primary role in the recommendation and prescription of any product candidates for which we may obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations, include the following:
| Ÿ | The federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under federally funded healthcare programs such as Medicare and Medicaid. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Violations of the federal anti-kickback statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. The Affordable Care Act clarified that a person or entity need not have actual knowledge of the federal anti-kickback statute or specific intent to violate it. In addition, the Affordable Care Act amended the federal civil False Claims Act to provide that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. There are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny. |
98
| Ÿ | The federal civil False Claims Act imposes civil penalties, and provides for whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, claims for payment of government funds that are false or fraudulent or knowingly making, or causing to be made, a false record or statement material to a false or fraudulent claim to avoid, decrease or conceal an obligation to pay money to the federal government. Several pharmaceutical and other healthcare companies have faced enforcement actions under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, federal anti-kickback statute violations and certain marketing practices, including off-label promotion, may also implicate the federal civil False Claims Act. Federal civil False Claims Act violations may result in civil monetary damages and penalties and exclusion from participation in federal healthcare programs. There are also criminal penalties, including imprisonment and criminal fines, for making or presenting a false, fictitious or fraudulent claim to the federal government. |
| Ÿ | The federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. |
| Ÿ | The federal criminal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact, making any materially false, fictitious, or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services. |
| Ÿ | The federal Physician Payment Sunshine Act, being implemented as the Open Payments Program, requires applicable pharmaceutical manufacturers of covered drugs to engage in extensive tracking of physician and teaching hospital payments, maintenance of a payments database, and public reporting of the payment data. Pharmaceutical manufacturers with products for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program were required to begin such tracking on August 1, 2013, and must make their first report to CMS by March 31, 2014 and annually thereafter. CMS will post manufacturer disclosures on a searchable public website. Failure to comply with the reporting obligations may result in civil monetary penalties. |
| Ÿ | Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by Medicaid or other state programs or, in several states, apply regardless of the payor. Several state laws require pharmaceutical companies to report expenses relating to the marketing and promotion of pharmaceutical products in those states and to report gifts and payments to individual health care providers in those states. Some of these states also prohibit certain marketing related activities including the provision of gifts, meals or other items to certain health care providers. In addition, California, Connecticut, Nevada, and Massachusetts require pharmaceutical companies to implement compliance programs or marketing codes. |
Regulation of preclinical research services
Preclinical research to support FDA submissions is subject to Good Laboratory Practices, or GLP, regulation and as a result the services we provide to third parties are subject to these regulations. Non-compliance with GLP can result in disqualification of the testing facility, and allows FDA to ignore the
99
results of any study conducted by the disqualified facility. Although we do not directly conduct animal studies, such studies which we may facilitate or contract to third parties are subject to GLP and the Animal Welfare Act which among other things sets minimum standards of care for certain animals used in research. The Animal and Plant Health Inspection Service of the U.S. Department of Agriculture administers the Animal Welfare Act.
Intellectual Property
We strive to protect the proprietary technology that we believe is important to our business, including seeking and maintaining patents intended to cover our product candidates and compositions, their methods of use and processes for their manufacture and any other inventions that are commercially important to the development of our business. We also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection.
As of January 14, 2014, we are the owner of 15 issued U.S. patents and 151 issued non-U.S. patents with claims to novel compounds, compositions containing them, processes for their preparation, their uses as pharmaceutical agents and test methods, with terms expiring between 2016 and 2030. Of these patents, one U.S. patent relates to SCY-078. We are actively pursuing nine U.S. patent applications (provisional and non-provisional), one international (PCT) patent application and 86 non-U.S. patent applications in at least 35 jurisdictions.
We are the exclusive licensee from Aventis Pharma of two issued U.S. patents and 63 issued non-U.S. patents, with claims to novel compounds, compositions containing them, processes for their preparation, and their uses as pharmaceutical agents, with terms expiring between 2017 and 2019. These include patents covering our clinical candidate SCY-635.
Our success will depend significantly on our ability to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how related to our business, defend and enforce our patents, maintain our licenses to use intellectual property owned by third parties, preserve the confidentiality of our trade secrets and operate without infringing the valid and enforceable patents and other proprietary rights of third parties. We also rely on know-how, continuing technological innovation and in-licensing opportunities to develop, strengthen, and maintain our proprietary position in the field of anti-fungal agents.
We believe that we have a strong intellectual property position and substantial know-how relating to the development and commercialization of SCY-078, consisting of patents or patent applications that we have co-invented with Merck. We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting our technology.
Our objective is to continue to expand our intellectual property estate by filing patent applications directed to SCY-078 and derivatives thereof, our cyclophilin platform and our contract research and development services. We intend to pursue, maintain, and defend patent rights, whether developed internally or licensed from third parties, and to protect the technology, inventions, and improvements that are commercially important to the development of our business.
SCY-078
The patent portfolio for SCY-078 is directed to cover compositions of matter, formulation, methods of use and precursors or intermediaries in its preparation. This patent portfolio includes an issued U.S. patent and corresponding foreign national and regional counterpart patents and patent applications. The patents and
100
patent applications relating to SCY-078 include patents and patent applications which were initially assigned to us and Merck Sharpe & Dohme Corp, a subsidiary of Merck & Co., Inc. We subsequently became the world-wide exclusive owner of Merck’s interests in SCY-078 for all applications. The issued composition of matter patent (U.S. Patent No. 8,188,085), if the appropriate maintenance, renewal, annuity, and other governmental fees are paid, is expected to expire in 2030. Based on our current development plan, we believe that an additional term of up to five years for the SCY-078 U.S. patent may result from the patent term extension provision of the Drug Price Competition and Patent Term Restoration Act of 1984 (the Hatch-Waxman Act). We expect that the patent applications in this portfolio, if issued, and if appropriate maintenance, renewal, annuity, and other governmental fees are paid, would expire between 2029 and 2035, including any additional term from patent term adjustment or patent term extension. The patent term calculation method and the provisions under the Hatch-Waxman Act are described in the “Patent Term” section below. We are not currently aware of any third-party patents (other than patents we have licensed) encompassing SCY-078.
The terms of issued SCY-078 composition of matter patents in other jurisdictions (Armenia, Azerbaijan, Belarus, Lebanon, Kazakhstan, Kyrgyzstan, Mexico, Moldova, New Zealand, Russia, Singapore, South Africa, Tajikistan, Turkmenistan,) if the appropriate maintenance, renewal, annuity, and other government fees are paid, are expected to expire in 2029. These patents and patent applications (if applicable), depending on the national laws, may benefit from extension of patent term in individual countries. In some European countries, for example, a supplementary protection certificate, if obtained, provides a maximum five years of market exclusivity. The duration of the supplementary protection certificate may be extended to five and a half years when the supplementary protection certificate relates to a human medicinal product for which data from clinical trials conducted in accordance with an agreed Pediatric Investigation Plan, or PIP, have been submitted. Likewise, in Japan, the term of a patent may be extended by a maximum of five years in certain circumstances.
SCY-641
The patent portfolio for SCY-641 is directed to cover compositions of matter, formulation, and methods of use. This patent portfolio includes issued U.S. patents and corresponding foreign national and regional counterpart patents and patent applications. The patents and patent applications relating to SCY-641 include patents and patent applications owned by us. The issued composition of matter patent (U.S. Patent No. 6,583,265), if the appropriate maintenance, renewal, annuity, and other government fees are paid, is expected to expire in 2019. The issued methods of use patents (U.S. Patent Nos. 8,188,052 and 8,551,952), if the appropriate maintenance, renewal, annuity, and other government fees are paid, are expected to expire in 2029 or 2027, respectively. We believe that the term for up to five years for one of the SCY-641 U.S. patents may be extended under the patent term extension provision of the Hatch-Waxman Act. We expect that the patent applications in this portfolio, if issued, and if appropriate maintenance, renewal, annuity, and other governmental fees are paid, would expire between 2019 and 2034, including any additional term from patent term adjustment or patent term extension, assuming that five year extension is granted. The patent term calculation method and the provisions under the Hatch-Waxman Act are described in the “Patent Term” section below.
The term of issued SCY-641 composition of matter patents in other jurisdictions (Australia, Canada, China, Europe and Japan) and methods of use patents and patent applications (if applicable) relating to SCY-641 (in Australia, Canada, China, Europe, Japan and South Africa), if the appropriate maintenance, renewal, annuity, and other government fees are paid, are expected to expire between 2019 and 2027. The patents and patent applications (if applicable), covering SCY-641, depending on the national laws, may also benefit from extension of patent term in individual countries.
101
Other product candidates
In addition to SCY-078, SCY-635 and SCY-641, we have a chemical library of more than 1,000 macrocyclic compounds generated by the research team at SCYNEXIS. This library includes compounds which are covered by patents or patent applications filed by us, but also includes novel chemical compounds which could form the basis for future patent applications.
Patent Term
The term of individual patents and patent applications will depend upon the legal term of the patents in the countries in which they are obtained. Generally, the patent term is 20 years from the date of filing of the patent application (or parent application, if applicable).
Under the Hatch-Waxman Act, the term of a patent that covers an FDA-approved drug may also be eligible for patent term extension, or PTE. PTE permits patent term restoration of a U.S. patent as compensation for patent term lost during the FDA regulatory review process. The length of the patent term extension is related to the length of time the drug is under regulatory review. The Hatch-Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent; however, a patent term extension cannot in any event extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions may be available in Europe and certain other foreign jurisdictions to extend the term of a patent that covers an approved drug. When possible, depending upon the length of clinical trials and other factors involved in the filing of an NDA we expect to apply for patent term extensions for patents covering SCY-078 and its use in treating various diseases. As a specific example, if we are awarded the maximum length of PTE, our U.S. granted composition of matter patents relating to SCY-078 would have an expected expiration date of August 28, 2035. However, depending on any changes in our clinical path and the date of FDA approval, the PTE may not be granted, or may be less than the maximum.
Proprietary rights and processes
We may rely, in some circumstances, on proprietary technology and processes (including trade secrets) to protect our technology. However, these can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors, contractors, and collaborators. We also seek to preserve the integrity and confidentiality of our proprietary technology and processes by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our proprietary technology and processes may otherwise become known or be independently discovered by competitors. To the extent that our employees, consultants, scientific advisors, contractors, or collaborators use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. For this and more comprehensive risks related to our proprietary technology and processes, please see the section on “Risk Factors—Risks Relating to Our Intellectual Property.”
Legal Proceedings
From time to time, we are involved in various legal proceedings arising in the ordinary course of our business. We are not currently a party to any legal proceedings the outcome of which, if determined adversely to us, would individually or in the aggregate have a material effect on our business, operating results or financial condition.
102
Employees
As of September 30, 2013, we had 90 employees, all of whom were employed on a full-time basis. Our employees are engaged in administration, finance, clinical development, regulatory, manufacturing, sales and marketing, and business development functions. 38 of our employees have Ph.D. degrees in the sciences and are focused on human and animal drug development. We believe our relations with our employees are good.
Facilities
Our corporate headquarters are located in Durham, North Carolina in a leased facility consisting of approximately 90,000 square feet of office space. The lease for this facility expires in March 2019, and includes a renewal option to extend the lease through March 2024.
103
Directors and Officers
The following table sets forth information regarding our directors and officers:
| Name |
Age | Position | ||||
| Yves J. Ribeill, Ph.D.* |
54 | President, Chief Executive Officer and Director | ||||
| Carole Sable, MD.* |
52 | Chief Medical Officer | ||||
| Charles F. Osborne, Jr.* |
48 | Chief Financial Officer | ||||
| Eileen C. Pruette* |
55 | General Counsel | ||||
| Vivian W. Doelling, Ph.D.* |
58 | Vice President of Animal Health | ||||
| Michael Garrett |
49 | Vice President of Corporate and Strategic Development | ||||
| Amanda S. Mancuso |
41 | Chief of Staff | ||||
| Pamela J. Kirby, Ph.D. |
60 | Chairman of our Board of Directors | ||||
| Laurent Arthaud |
51 | Director | ||||
| Mounia Chaoui, Ph.D. |
42 | Director | ||||
| Ann F. Hanham, Ph.D. |
61 | Director | ||||
| Patrick J. Langlois, Ph.D. |
68 | Director | ||||
| Jean-Yves Nothias, Ph.D. |
52 | Director | ||||
| Edward E. Penhoet, Ph.D. |
73 | Director | ||||
| * | Executive Officer |
| (1) | Member of the audit committee |
| (2) | Member of the compensation committee |
| (3) | Member of the nominating and corporate governance committee |
Executive Officers and Other Key Employees
Yves J. Ribeill, Ph.D. Dr. Ribeill has served as our President and Chief Executive Officer and a member of our board of directors since November 1999. From 1982 to 2000, Dr. Ribeill held various positions during a 20-year international pharmaceutical career with Aventis Pharma S.A. and its predecessor Rhône-Poulenc Rorer. His roles with those companies included Discovery Chemistry Group Leader for Anti-Viral Research. He also served as a member of the Central Nervous System Group and as Director of Chemistry for the Anti-Infective Group. He was involved in all phases of the drug discovery and development effort that resulted in FDA approval of the anti-bacterial Synercid in 1999. Dr. Ribeill is the author of 24 scientific publications and 15 patents. He was a member of the Scientific Advisory Committee of the World Health Organization. Dr. Ribeill has a Ph.D. in Chemistry from the University of Montpellier in France. Because of Dr. Ribeill’s extensive knowledge of our company, the pharmaceutical industry and our competitors, we believe he is able to make valuable contributions to our board of directors.
Carole Sable, MD. Dr. Sable joined us as our Chief Medical Officer in January 2014. Before joining the company, Dr. Sable was a Vice President at Merck & Co., Inc, from 2010 to 2013, initially in the Infectious Disease franchise, where she was responsible for coordinating cross functional activities of the discovery and early development programs, and then in the Neurosciences and Ophthalmology franchise, where she was VP in the Project Leadership and Management group, overseeing cross functional activities in late development programs. Dr. Sable served as Chief Medical Officer of Novexel SA and President of Novexel Inc., the US subsidiary, from 2007 to 2010, where she was responsible for clinical development and successfully filed two investigational new drug applications and successfully completed Phase 2b
104
studies for two antibacterial programs which led to the acquisition of Novexel SA by AstraZeneca. Prior to her position as Chief Medical Officer at Novexel, Dr. Sable was with Merck & Co., Inc. from 1995 to 2007, serving in various capacities in Infectious Disease and Vaccines Clinical Development, ultimately becoming Executive Director in 2006, where she was responsible for anti-bacterial, anti-fungal and vaccine development programs, including the clinical development of the anti-fungal agent Cancidas®. While with Merck, Dr. Sable also supervised the development and regulatory submissions for Invanz® and the in-licensing of the anti-infective amino-methylcycline PTK-0796 from Paratek Pharmaceuticals, as well as programs for sepsis, malaria, and anthrax. Prior to joining Merck, Dr. Sable was an Assistant Professor of Medicine and Infectious Diseases at the University of Virginia in Charlottesville. She received an MD from Jefferson Medical College in 1983 and completed an internal medicine residency and infectious disease fellowship at the University of Virginia.
Charles F. Osborne, Jr. Mr. Osborne, a certified public accountant, has served as our Chief Financial Officer since November 2003. From 1999 to 2003, he was Chief Financial Officer of Nobex Corporation in Durham, North Carolina. At Nobex, Mr. Osborne completed two venture capital rounds totaling more than $60 million. He also was involved in structuring and negotiating corporate licenses and research agreements with global pharmaceutical companies, including GlaxoSmithKline plc. From 1992 to 1998, he was Vice President of Finance for International Murex Technologies Co. While at Murex, he ran the worldwide finance group while based in London and was involved with the sale of the company to Abbott Laboratories. He holds a B.S. in Accounting from the University of North Carolina at Chapel Hill.
Eileen C. Pruette. Ms. Pruette has served as our General Counsel since August 2012. From 2010 to 2012, Ms. Pruette served as Counsel to the U.S. commercial operations of bioMerieux SA (EPA: BIM), a multinational biotechnology company headquartered in France. From 2003 to 2008, she served as General Counsel for Valeant Pharmaceuticals International, Inc. (TSE: VRX), a multinational specialty pharmaceutical company. From 2001 to 2003, Ms. Pruette served as the Vice President of U.S. Legal and Global Intellectual Property of the Sony Ericsson Mobile Communications joint venture. From 1996 to 2001, she served as Division Counsel for the U.S. operations of Telefonaktiebolaget L. M. Ericsson. From 1990 to 1996 Ms. Pruette served as Corporate Counsel at GlaxoSmithKline plc (then Glaxo, Inc.). Prior to joining Glaxo, Ms. Pruette was an associate with Moore & Van Allen PLLC, a law firm, in Durham, North Carolina. She has a B.S. in Business Administration from the University of North Carolina at Chapel Hill and received her law degree from the Van Hecke-Wettach School of Law at the University of North Carolina at Chapel Hill.
Vivian W. Doelling, Ph.D. Dr. Doelling has served as our Vice President of Animal Health since October 2013. From 2011 until 2013, Dr. Doelling was a Senior Scientist at Integrated Laboratory Systems, Inc., a multidisciplinary research organization, where she was responsible for providing scientific support for biological and toxicological test method evaluation. From 2009 to 2011, she was an independent consultant to agricultural biotechnology and animal health industries. From 1992 to 2009, Dr. Doelling held various positions at Embrex, Inc., including Vice President of Research and Development where she managed a $9 million budget and more than 40 scientists. From 1990 to 1991, Dr. Doelling was the Biochemistry Group Leader for the medical research division of American Cyanamid Company. She received her B.S. in Biology from Dickinson College and her Ph.D. in Biological Sciences from Purdue University.
Michael Garrett. Mr. Garrett has served as our Vice President of Corporate and Strategic Development since May 2006. From 2004 to 2006, he was a Managing Director of Pharmavent Partners, a European life sciences venture capital fund headquartered in Paris. At Pharmavent, Mr. Garrett was responsible for UK-based investment opportunities. From 2001 to 2004, he was Global Vice President of Ventures and Business Development for BTG plc. While at BTG, Mr. Garrett was responsible for a portfolio of 15 investments in early stage to public companies in Canada, the United Kingdom and the United States. He is
105
a British and European Patent Attorney, holds an Honors Chemistry degree from Southampton University, United Kingdom and an Executive Certificate in General Management from the Cedep-INSEAD business school in France.
Amanda S. Mancuso. Ms. Mancuso has served as our Chief of Staff since January 2012. Ms. Mancuso served as our Executive Director of Human Resources from 2006 to 2011 and as our Director of Human Resources from 2001 to 2006. From 1998 to 2000, she was the Head of Expatriate Services at Rhône-Poulenc Ag Company in Durham, North Carolina. In this role, she managed the international assignments of high potential employees being developed for larger roles within the organization. From 1994 to 1998, she held various positions in human resources and public relations with Rhône-Poulenc Ag Company. Ms. Mancuso holds a B.A. from Appalachian State University and an M.B.A. from Duke University.
Non-Employee Directors
Pamela J. Kirby, Ph.D. Dr. Kirby has served as the Chairman of our board of directors since January 2006 and has served as a director since December 2004. She brings over 25 years of experience in the pharmaceutical and biotechnology industries. Dr. Kirby served as a director of Novo Nordisk A/S, a global healthcare company, from 2008 to 2011 and as a member of the board of Simmons & Simmons LLP, an international law firm, from 2011 to 2013. She has served as a director of Smith and Nephew plc (LSE: SN), a multinational medical equipment manufacturing company, since 2002, Informa plc (LSE: INF), a multinational publishing and conference company, since 2004, Victrex plc (LSE: VCT), a producer of high performance polymers, since 2011 and DCC plc, a diversified investments group headquartered in Ireland, since 2013. From 2001 to 2003, Dr. Kirby was the Chief Executive Officer of Quintiles Transnational Corporation. From 1998 to 2001, she served as Director of Global Strategic Marketing and Business Development in the pharmaceutical division of Hoffmann-La Roche Ltd. From 1996 to 1998, she served as Commercial Director at British Biotech plc (now Vernalis plc). From 1979 to 1996, Dr. Kirby was with Astra AB (now AstraZeneca AB), rising through various senior management positions, being named Vice President of Corporate Strategy, Marketing and Business Development in 1994. She has a BSc in Pharmacology and a Ph.D. in Clinical Pharmacology from the University of London. Because of Dr. Kirby’s experience in senior executive positions within pharmaceutical and clinical research organizations and her extensive board experience we believe she is able to make valuable contributions to our board of directors.
Laurent Arthaud. Mr. Arthaud has served as a member of our board of directors since April 2007. Since 2006 he has served as a General Partner with Bpifrance Investissement, formerly CDC Entreprises, a private equity firm based in Paris, responsible for investments in the biotech field. From 2004 to 2006, he was managing partner with Pharmavent Partners, also headquartered in Paris, and from 1999 to 2004, Mr. Arthaud was in charge of the venture capital activities of Aventis and managed the venture capital fund F.C.P.R. Genavent. Mr. Arthaud started his career in 1986 at the INSEE (French Economic Statistics Institute), and then at the Forecasts Department of the French Ministry of Finances. In 1995, he joined the cabinet of French Prime Minister Alain Juppé as Technical Advisor in charge of workforce and unemployment matters. He joined Rhône-Poulenc Group in 1997 as Scientific Board General Secretary. Mr. Arthaud is a graduate from the Ecole Polytechnique of Paris and from the Ecole Nationale de la Statistique et de l’Administration Economique. Because of Mr. Arthaud’s extensive experience, both in the pharmaceutical industry and in the domain of investments in biotechnology companies, we believe he is able to make valuable contributions to our board of directors.
Mounia Chaoui, Ph.D. Dr. Chaoui has served as a member of our board of directors since January 2012. Since May 2013, Dr. Chaoui has served as a General Partner at Turenne Capital Partenaires, a private equity and venture capital firm, and from January 2012 to December 2012, she served as a Managing Partner at Inserm Transfert Initiative, a private subsidiary of the French National Institute of Health and
106
Medical Research. From 2001 to 2011, Dr. Chaoui was a General Partner at Ventech Capital. From 1999 to 2001, she served as a consultant to Altran Technologies, where she conducted strategic audits, performed due diligence procedures on behalf of investors and was involved in fundraising for several start-up companies. From 1998 to 1999, was Dr. Chaoui was a member of the life sciences team at Atlas Venture, and from 1995 to 1998, was a Ph.D. student with the Gustav Roussy Institute. Dr. Chaoui served as a member of the board of directors of Cellerix (EUR: TIG) from 2007 to 2012, Funxional Therapeutics from 2011 to 2012 (acquired by Boehringer Ingelheim GmbH) and BioVex Group Inc. from 2009 to 2011 (acquired by Amgen, Inc. in 2011). Currently, she is member of the supervisory boards of ActoGeniX NV, Covagen AG, Eyegate Pharmaceuticals, Inc., Prosonix Ltd. and Groupe SEBBIN SAS. Dr. Chaoui graduated as a bioengineer from École Centrale de Paris and holds a Ph.D. in molecular biophysics from University of Paris VI. Because of Dr. Chaoui’s extensive experience in the life sciences venture capital industry, we believe she is able to make valuable contributions to our board of directors.
Ann F. Hanham, Ph.D. Dr. Hanham has served as a member of our board of directors since December 2008. From 2000 to 2013, Dr. Hanham served with Burrill & Company, a life sciences venture capital firm, becoming a Managing Director and General Partner in 2006. From 1998 to 2000, Dr. Hanham was a co-founder and Vice President of Clinical & Regulatory Affairs at InterMune, Inc. From 1995 to 1998, she served as the Senior Director for Oncology Product Development at Otsuka Pharmaceuticals and from 1991 to 1995 as the Medical Director for Celtrix Pharmaceuticals. From 1988 to 1991, Dr. Hanham worked for Becton Dickinson in both regulatory and clinical affairs for the monoclonal antibody program, and from 1984 to 1988 as a regulatory toxicologist with the Health Protection Branch of Health and Welfare Canada. She has also served as a member of the board of directors of Adlyfe Inc. since 2006, Acusphere, Inc. since 2013, Endocyte, Inc. (NASDAQ: ECYT) since 2004, and Waterstone Pharmaceuticals, Inc. since 2008. Dr. Hanham holds a Ph.D. from the University of British Columbia, an MSc from Simon Fraser University, and a BSc from the University of Toronto. She was also Board Certified in Toxicology in 1986. Because of Dr. Hanham’s extensive clinical and regulatory experience, as well as her extensive experience in working with development stage biotechnology companies, we believe she is able to make valuable contributions to our board of directors.
Patrick J. Langlois, Ph.D. Dr. Langlois has served as a member of our board of directors since April 2006. Since March 2005, Dr. Langlois has served as the General Partner of PJL Conseils, a consulting firm specializing in strategy, corporate development and mergers and acquisitions. From 2002 to 2004, he served as Vice Chairman of the Management Board and Chief Financial Officer at Aventis S.A., and from 1999 to 2002 as its Executive Vice President and Chief Financial Officer. At Aventis, Dr. Langlois was responsible for finance and corporate development functions, as well as three global businesses: dermatology, protein therapeutics and animal health. From 1990 to 1999, Dr. Langlois was employed by Rhône-Poulenc Group, most recently as Chief Financial Officer and a Member of the Executive Committee. From 1990 to 1996, he was employed by Rhône-Poulenc Rorer, a NYSE-listed pharmaceutical company, most recently as Chief Financial Officer. Dr. Langlois received a License degree from the University of Rennes, a Ph.D. degree in Economics from the University of Rennes and was awarded a Diploma in Higher Banking Studies from the Centre d’Etudes Supérieures de Banque in France. Because of Dr. Langlois’ extensive experience in the healthcare sector, including an executive position as chief financial officer of a NYSE-listed company as well as his relationships with institutional investors and investment banks in the United States and Europe, we believe he is able to make valuable contributions to our board of directors.
Jean-Yves Nothias, Ph.D. Dr. Nothias has served as a member of our board of directors since August 2000. Since 2012, Dr. Nothias has served as a Director of Genomic Vision SA, a biotechnology company headquartered in Paris. Since 2012, Dr. Nothias is Founder and President of a fund management company Vesale Partners, managing its Biotechnology Fund. From 2000 to 2011, Dr. Nothias served as a Managing Director of SG Asset Management, where he headed the Venture Capital Biotechnology Team. Since 2005,
107
he has served as a director of GenomeQuest Inc., and since 2012 he has served as a director of Bioforce Nanoscience Inc. Since 2009 he has served as an observer of the boards of directors of Somalogic Inc. and Pulmagen Therapeutics. From 1999 to 2000, he was a biotechnology corporate analyst for Oddo & Cie, a French brokerage firm. From 1996 to 1998, he was a sales side biotechnology analyst for Hambrecht & Quist based in Paris. Dr. Nothias holds a thesis in Molecular Biology from Université Pierre & Marie Curie and a master’s degree in management from Université Paris Sorbonne. Because of Dr. Nothias’s extensive biotechnology fund manager and board member experience, we believe he is able to make valuable contributions to our board of directors.
Edward E. Penhoet, Ph.D. Dr. Penhoet has served as a member of our board of directors since June 2002. Since 2000, he has served as a Director of Alta Partners, a life sciences venture capital firm. Since 2009, he has served on President Obama’s Council of Advisors on Science and Technology, an advisory group comprising 20 of the nation’s leading scientists and engineers who directly advise the President and the Executive Office of the President. From 2005 to 2010, he served as Vice-Chair of the governing board of the Independent Citizens Oversight Committee for the California Institute of Regenerative Medicine. From 2004 to 2008, he served as the President of the Gordon and Betty Moore Foundation. From 1998 to 2002, he served as the Dean of the School of Public Health at the University of California at Berkeley. Dr. Penhoet was a co-founder of Chiron Corporation, where he served as President and Chief Executive Officer from 1981 to 1998. From 1971 to 1981, he was a faculty member of the Biochemistry Department of the University of California at Berkeley. Dr. Penhoet has served as a member of the board of directors of Cymabay Therapeutics, Inc. since 2004, and served as a member of the boards of directors of ChemoCentryx, Inc (NASDAQ: CCXI) from 2007 to 2013, Corcept Therapeutics Incorporated (NASDAQ: CORT) from 2008 to 2010 and ZymoGenetics, Inc. (NASDAQ: ZGEN) from 2000 to 2010. He is a member of both the Institute of Medicine of the National Academies and the American Academy of Arts and Sciences and has co-authored more than 50 scientific articles and papers. Dr. Penhoet earned his A.B. in Biology from Stanford University and his Ph.D. in Biochemistry from the University of Washington. He was a post-doctoral fellow at the University of California, San Diego, from 1968 to 1970. Because of Dr. Penhoet’s extensive experience as an investor in life science companies, we believe he is able to make valuable contributions to our board of directors.
Each of our executive officers serves at the discretion of our board of directors and holds office until his or her successor is duly elected and qualified or until his or her earlier resignation or removal. There are no family relationships among any of our directors or executive officers.
Board Composition and Election of Directors
Our business and affairs are managed under the direction of our board of directors, which currently consists of eight members. The members of our board of directors were elected in compliance with the provisions of our amended and restated certificate of incorporation and a voting agreement among certain of our stockholders, as amended. The voting agreement will terminate upon the closing of this offering and none of our stockholders will have any special rights regarding the election or designation of members of our board of directors.
Director Independence
Under the listing requirements and rules of the NASDAQ Global Market, independent directors must compose a majority of our board of directors within a specified period of the closing of this offering.
Our board of directors has undertaken a review of its composition, the composition of its committees, and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment, and affiliations, including family relationships, our
108
board of directors has determined that all members of our board of directors except Dr. Ribeill do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under the applicable rules and regulations of the SEC and the listing requirements and rules of the NASDAQ Global Market. In making this determination, our board of directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director.
Board Committees
Our board of directors has the authority to appoint committees to perform certain management and administration functions. Upon the closing of this offering, our board of directors will have an audit committee, a compensation committee and a nominating and corporate governance committee. The composition and responsibilities of each committee are described below. Members will serve on these committees until their resignation or until otherwise determined by our board of directors. Following the closing of this offering, the charters for each of these committees will be available on our website at www.scynexis.com.
Audit Committee
Our audit committee currently consists of , and . Immediately following the closing of this offering, our audit committee will consist of , and , each of whom satisfies the independence requirements under the NASDAQ Global Market listing standards and Rule 10A-3(b)(1) of the Securities Exchange Act of 1934, or the Exchange Act. The chairperson of our audit committee is , whom our board of directors has determined to be an “audit committee financial expert” within the meaning of SEC regulations. Each member of our audit committee can read and understand fundamental financial statements in accordance with audit committee requirements. In arriving at this determination, our board of directors has examined each audit committee member’s scope of experience and the nature of their employment in the corporate finance sector.
Our audit committee oversees our corporate accounting and financial reporting process. The audit committee has the following responsibilities, among others things, as set forth in the audit committee charter:
| Ÿ | reviewing and pre-approving the engagement of our independent registered public accounting firm to perform audit services and any permissible non-audit services; |
| Ÿ | evaluating the performance of our independent registered public accounting firm and deciding whether to retain their services; |
| Ÿ | reviewing our annual and quarterly financial statements and reports and discussing the statements and reports with our independent registered public accounting firm and management, including a review of disclosures under the section of this prospectus titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations;” |
| Ÿ | considering and approving or disapproving of all related party transactions; |
| Ÿ | preparing the audit committee report required by the SEC to be included in our annual proxy statement; |
| Ÿ | reviewing, with our independent registered public accounting firm and management, significant issues that may arise regarding accounting principles and financial statement presentation, as well as matters concerning the scope, adequacy and effectiveness of our financial controls; |
109
| Ÿ | conducting an annual assessment of the performance of the audit committee and its members, and the adequacy of its charter; and |
| Ÿ | establishing procedures for the receipt, retention and treatment of complaints received by us regarding financial controls, accounting or auditing matters. |
Compensation Committee
Our compensation committee currently consists of , and . The chairperson of our compensation committee is . Immediately following the closing of this offering, our compensation committee will consist of , and , each of whom our board of directors has determined to be independent under the NASDAQ Global Market listing standards, a “non-employee director” as defined in Rule 16b-3 promulgated under the Exchange Act, and an “outside director” as that term is defined in Section 162(m) of the Internal Revenue Code.
Our compensation committee reviews and recommends policies relating to compensation and benefits of our officers and employees. The compensation committee has the following responsibilities, among other things, as set forth in the compensation committee’s charter:
| Ÿ | determining the compensation and other terms of employment of our chief executive officer and our other executive officers and reviewing and approving corporate performance goals and objectives relevant to the compensation; |
| Ÿ | reviewing and recommending to the full board of directors the compensation of our non-employee directors; |
| Ÿ | evaluating, adopting and administering the equity incentive plans, compensation plans, and similar programs advisable for us, as well as modification or termination of existing plans and programs; |
| Ÿ | establishing policies with respect to equity compensation arrangements; |
| Ÿ | reviewing and discussing annually with management our “Compensation Discussion and Analysis” if required by SEC rules; |
| Ÿ | preparing the compensation committee report if required by the SEC to be included in our annual proxy statement; and |
| Ÿ | reviewing and evaluating, at least annually, the performance of the compensation committee and the adequacy of its charter. |
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee currently consists of , and . Immediately following the closing of this offering, our nominating and corporate governance committee will consist of , and , each of whom our board of directors has determined to be independent under the NASDAQ Global Market listing standards. The chairperson of our nominating and corporate governance committee is .
Our nominating and corporate governance committee makes recommendations regarding corporate governance, the composition of our board of directors, identification, evaluation and nomination of director candidates and the structure and composition of committees of our board of directors. The nominating and corporate governance committee has the following responsibilities, among other things, as set forth in the nominating and corporate governance committee’s charter:
| Ÿ | reviewing periodically and evaluating director performance on our board of directors and its applicable committees, and recommending to our board of directors and management areas for improvement; |
110
| Ÿ | interviewing, evaluating, nominating and recommending individuals for membership on our board of directors; |
| Ÿ | reviewing and recommending to our board of directors any amendments to our corporate governance policies; and |
| Ÿ | reviewing and assessing, at least annually, the performance of the nominating and corporate governance committee and the adequacy of its charter. |
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. Following the closing of this offering, the code of business conduct and ethics will be available on our website at www.scynexis.com. We will disclose any amendments to the code, or any waivers of its requirements, on our website to the extent required by the applicable rules and exchange requirements.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee has ever been an officer or employee of our company. None of our executive officers serve, or have served during the last fiscal year, as a member of our board of directors, compensation committee or other board committee performing equivalent functions of any entity that has one or more executive officers serving as one of our directors or on our compensation committee.
Director Compensation
We currently do not provide cash compensation to certain of our non-employee directors. From time to time, we have granted stock options to certain of our non-employee directors as compensation for their services. Dr. Ribeill, who is also an employee, is compensated for his service as an employee and does not receive any additional compensation for his service on our board of directors.
The following table sets forth information regarding compensation earned by our non-employee directors during the fiscal year ended December 31, 2013.
| Name |
Cash compensation | Option awards(1) |
All other compensation |
Total | ||||||||||||
| Pamela J. Kirby, Ph.D. |
— | $42,000 | $42,000 | |||||||||||||
| Laurent Arthaud |
— | $18,000 | $18,000 | |||||||||||||
| Mounia Chaoui, Ph.D. |
— | — | — | |||||||||||||
| Ann F. Hanham, Ph.D. |
— | — | — | |||||||||||||
| Patrick J. Langlois, Ph.D. |
— | $36,000 | $7,840 | $43,840 | ||||||||||||
| Jean-Yves Nothias, Ph.D. |
— | — | — | |||||||||||||
| Edward E. Penhoet, Ph.D. |
— | — | — | |||||||||||||
| (1) | The amounts in this column reflect the aggregate grant date fair value of each option award granted during the fiscal year, as computed in accordance with FASB ASC Topic 718. The grant date fair value of such option awards is $1.20. The valuation assumptions used in determining such amounts are described in Note 11 to our financial statements included in this prospectus. The table below lists the aggregate number of shares and additional information with respect to the outstanding option awards held by each of our non-employee directors. |
111
| Name |
Number of shares subject to outstanding options as of December 31, 2013(1) |
|||
| Pamela J. Kirby, Ph.D. |
308,000 | |||
| Laurent Arthaud |
90,000 | |||
| Mounia Chaoui, Ph.D. |
— | |||
| Ann F. Hanham, Ph.D. |
— | |||
| Patrick J. Langlois, Ph.D. |
205,000 | |||
| Jean-Yves Nothias, Ph.D. |
— | |||
| Edward E. Penhoet, Ph.D. |
— | |||
| (1) | Includes options to purchase 35,000 shares, 15,000 shares and 30,000 shares of our common stock that were granted to Dr. Kirby, Mr. Arthaud and Dr. Langlois, respectively, on December 20, 2013, under our 2009 Stock Option Plan, or 2009 Plan. |
Following the closing of this offering, we intend to compensate our non-employee directors with a combination of cash and equity. Each non-employee director will receive an annual base cash retainer of $30,000 for such service, to be paid quarterly. The chairman of our board of directors will receive an additional annual base cash retainer of $15,000 for this service, to be paid quarterly.
In addition, we intend to compensate the members of our board of directors for service on our committees as follows:
| Ÿ | The chairperson of our audit committee will receive an annual cash retainer of $10,000 for this service, paid quarterly, and each of the other members of the audit committee will receive an annual cash retainer of $6,500, paid quarterly. |
| Ÿ | The chairperson of our compensation committee will receive an annual cash retainer of $7,500 for such service, paid quarterly, and each of the other members of the compensation committee will receive an annual cash retainer of $5,000, paid quarterly. |
| Ÿ | The chairperson of our nominating and corporate governance committee will receive an annual cash retainer of $4,500 for this service, paid quarterly, and each of the other members of the nominating and corporate governance committee will receive an annual cash retainer of $3,000, paid quarterly. |
Further, after the closing of this offering, each year at about the time of our annual meeting of stockholders, each non-employee director will receive an additional equity award of an option to purchase 30,000 shares of our common stock, and our chairman will receive an additional equity award of an option to purchase 15,000 shares of our common stock. If a new board member joins our board of directors after the closing of this offering, the director will receive an initial stock option to purchase 65,000 shares of our common stock, and if a new chairman joins our board of directors after the closing of this offering, the chairman will receive an initial stock option to purchase 97,500 shares of our common stock. Annual option grants and option grants to new board members will vest in full on the earlier of our annual meeting of stockholders in the year following the date of grant or the one year anniversary of the date of grant.
112
Summary Compensation Table
The following table provides information regarding the compensation of our principal executive officer, principal financial officer and each of our two other executive officers during the fiscal year ended December 31, 2012. We refer to these executive officers in this prospectus as our named executive officers.
| Name and Principal Position |
Year | Salary | Bonus | Option awards(1) |
All other compensation |
Total | ||||||||||||||||||
| Yves J. Ribeill, Ph.D. |
2013 | $ | 250,146 | — | — | $ | 14,954 | (2) | $ | 265,101 | ||||||||||||||
| President and Chief Executive Officer |
2012 | $ | 250,203 | — | — | $ | 13,055 | $ | 263,258 | |||||||||||||||
| Charles F. Osborne, Jr. |
2013 | $ | 250,205 | — | — | $ | 5,819 | (3) | $ | 256,024 | ||||||||||||||
| Chief Financial Officer |
2012 | $ | 250,213 | — | — | $ | 8,779 | $ | 258,992 | |||||||||||||||
| Eileen C. Pruette(4) |
2013 | $ | 235,062 | — | — | $ | 8,477 | (5) | $ | 243,539 | ||||||||||||||
| General Counsel |
2012 | $ | 87,372 | — | $ | 180,000 | $ | 2,687 | $ | 270,059 | ||||||||||||||
| Vivian W. Doelling, Ph.D.(6) |
2013 | $ | 43,885 | — | — | $ | 1,603 | (7) | $ | 45,487 | ||||||||||||||
| Vice President of Animal Health |
||||||||||||||||||||||||
| (1) | The amounts in this column reflect the aggregate grant date fair value of each option award granted during the fiscal year, computed in accordance with FASB ASC Topic 718. The grant date fair value of such option award is $0.90. The valuation assumptions used in determining such amounts are described in Note 11 to our financial statements included in this prospectus. |
| (2) | Includes tax preparation payments in the amount of $6,000, short term/long term disability premiums in the amount of $1,030 and life insurance premiums in the amount of $420. Also includes $5,685 contributed to his 401(k) plan account. |
| (3) | Includes short term/long term disability premiums in the amount of $1,030 and life insurance premiums in the amount of $420. Also includes $4,369 contributed to his 401(k) plan account. |
| (4) | Ms. Pruette’s employment with us began in August 2012. |
| (5) | Includes short term/long term disability premiums in the amount of $1,030 and life insurance premiums in the amount of $420. Also includes $7,052 contributed to her 401(k) plan account. |
| (6) | Dr. Doelling’s employment with us began in October 2013. |
| (7) | Includes short term/long term disability premiums in the amount of $213 and life insurance premiums in the amount of $73. Also includes $1,317 contributed to her 401(k) plan account. |
113
Outstanding Equity Awards as of December 31, 2013
The following table provides information regarding outstanding equity awards held by our named executive officers as of December 31, 2013.
| Number of Securities Underlying Unexercised options |
Option exercise Price |
Option expiration Date |
||||||||||||||
| Name |
Exercisable(1) | Unexercisable | ||||||||||||||
| Yves J. Ribeill, Ph.D. |
150,000 | — | $ | 1.00 | 10/22/14 | |||||||||||
| 150,000 | — | $ | 1.00 | 04/28/15 | ||||||||||||
| 19,000 | — | $ | 1.00 | 04/20/16 | ||||||||||||
| 75,000 | — | $ | 1.00 | 04/26/17 | ||||||||||||
| 60,000 | — | $ | 1.00 | 04/18/18 | ||||||||||||
| 75,000 | — | $ | 1.25 | 04/23/19 | ||||||||||||
| 60,000 | $ | 1.27 | 07/14/20 | |||||||||||||
| 20,000 | 20,000 | (2) | $ | 1.50 | 04/20/21 | |||||||||||
| Charles F. Osborne, Jr. |
19,600 | — | $ | 1.00 | 10/22/14 | |||||||||||
| 19,074 | — | $ | 1.00 | 04/28/15 | ||||||||||||
| 10,000 | — | $ | 1.00 | 04/20/16 | ||||||||||||
| 25,000 | — | $ | 1.00 | 04/26/17 | ||||||||||||
| 16,500 | — | $ | 1.00 | 04/18/18 | ||||||||||||
| 25,000 | — | $ | 1.25 | 04/23/19 | ||||||||||||
| 30,000 | — | $ | 1.27 | 07/14/20 | ||||||||||||
| 8,500 | 8,500 | (2) | $ | 1.50 | 04/20/21 | |||||||||||
| Eileen C. Pruette |
42,640 | 157,360 | (3) | $ | 1.20 | 10/24/22 | ||||||||||
| Vivian W. Doelling, Ph.D. |
— | 100,000 | (4) | $ | 2.70 | 12/19/23 | ||||||||||
| (1) | The options listed are fully vested or are subject to an early exercise right and may be exercised in full prior to vesting of the shares underlying such options. Vesting of all options is subject to continued service on the applicable vesting date. |
| (2) | 25% of the shares subject to this option vested on April 21, 2012, 25% of the shares subject to this option vested on April 21, 2013 and 50% of the shares subject to this option vests on April 21, 2014. |
| (3) | 15% of the shares subject to this option vested on August 20, 2013, 1.58% of the shares subject to the option vest monthly for the next twelve months and 2.75% of the shares subject to the option vest monthly for 24 months thereafter. |
| (4) | 10% of the shares subject to this option vest on October 17, 2014, 0.83% of the shares subject to the option vest monthly for the next twenty-four months and 5.84% of the shares subject to the option vest monthly for twelve months thereafter. |
Change in Control Severance Benefits
We have entered into employment agreements with each of Dr. Ribeill, Ms. Pruette, Dr. Sable and Mr. Osborne that contain severance provisions providing for continued payment of salary and provision of benefits for a specified period of time in connection with termination of employment under various circumstances, including involuntary termination by us or termination by the employee for good reason.
The actual amounts that would be paid or distributed to an eligible executive officer as a result of a termination of employment occurring in the future may be different than those presented below, as many factors will affect the amount of any payments and benefits upon a termination of employment. For
114
example, some of the factors that could affect the amounts payable include the executive officer’s base salary and the market price of our common stock. Although we have entered into a written agreement to provide severance payments and benefits in connection with a termination of employment under particular circumstances, we may mutually agree with the executive officers to provide payments and benefits on terms that vary from those currently contemplated. In addition to the amounts presented below, each executive officer is eligible to receive any benefits accrued under our broad-based benefit plans, such as accrued vacation pay, in accordance with those plans and policies.
To receive any of the severance benefits under these agreements, the executive officer would be required to execute a release of claims against us and comply with further cooperation, confidentiality and noncompetition provisions.
Severance Payments
In the event of a termination without “just cause” by us or an executive officer’s resignation for “good reason” at any time during the period that is within twelve months following a “change in control,” which termination we refer to as a Change in Control Termination, the executive officer is eligible to receive the following payments and benefits:
| Ÿ | a cash amount equal to a portion (twelve months in the case of Ms. Pruette, Dr. Sable and Mr. Osborne or 24 months in the case of Dr. Ribeill) of the executive officer’s then current base salary, which shall be paid over twelve months (in the case of Ms. Pruette, Dr. Sable and Mr. Osborne) or 24 months (in the case of Dr. Ribeill) commencing with the first payroll period following the termination date; and |
| Ÿ | health insurance premiums under our group health insurance plans as provided under the Consolidated Omnibus Budget Reconciliation Act, or COBRA, until the earlier of (a) twelve months (in the case of Ms. Pruette, Dr. Sable and Mr. Osborne) or 24 months (in the case of Dr. Ribeill) after termination of employment, (b) such time as the executive officer becomes enrolled in the group health insurance plan of another employer or (c) the executive officer becomes entitled to Medicare after the COBRA election. |
In the event of a termination without “just cause” by us or an executive officer’s resignation for “good reason” at any time other than during the twelve month period following a “change in control,” which we refer to as a Covered Termination, the executive officer is eligible to receive the following payments and benefits:
| Ÿ | a cash amount equal to a portion (six months in the case of Ms. Pruette, Dr. Sable and Mr. Osborne or twelve months in the case of Dr. Ribeill) of the executive officer’s then current base salary, which shall be paid over six months (in the case of Ms. Pruette, Dr. Sable and Mr. Osborne) or twelve months (in the case of Dr. Ribeill) commencing with the first payroll period following the termination date; and |
| Ÿ | health insurance premiums under our group health insurance plans as provided under COBRA, until the earlier of (a) six months (in the case of Ms. Pruette, Dr. Sable and Mr. Osborne) or twelve months (in the case of Dr. Ribeill) after termination of employment, (b) such time as the executive officer becomes enrolled in the group health insurance plan of another employer or (c) the executive officer becomes entitled to Medicare after the COBRA election. |
Equity Awards
In the event of a Change in Control Termination, the vesting and exercisability of all outstanding options to purchase our common stock held by an eligible executive officer will be accelerated in full, and any repurchase rights held by us respect to our common stock issued or issuable pursuant to any other stock award granted to such executive officer will lapse.
115
In the event of a Covered Termination, the vesting and exercisability of all outstanding options to purchase our common stock held by an eligible executive officer will be accelerated, and any repurchase rights held by us with respect to our common stock issued or issuable pursuant to any other stock award granted to such executive officer will lapse, with respect to the same number of shares if the executive officer had continued employment for an additional six months (in the case of Ms. Pruette, Dr. Sable and Mr. Osborne) or twelve months (in the case of Dr. Ribeill).
For purposes of these agreements, the term “change in control” means the occurrence of any of the following: (a) our company being party to any merger, consolidation or other similar transaction that results in our stockholders immediately before the merger, consolidation or other similar transaction owning less than 50% of the equity, or possessing less than 50% of the voting control, of us or the successor entity in the merger, consolidation or similar transaction; (b) any liquidation, dissolution or other sale or disposition of all or substantially all of our assets; or (c) our stockholders sell or otherwise dispose of our capital stock in a single transaction or series of related transactions such that the stockholders immediately before such transaction or related transactions own less than 50% of the equity, and possess less than the voting power, of our capital stock; provided, however, that an initial public offering or subsequent public offering of our common stock does not constitute a “change in control.”
For purposes of these agreements, the term “just cause” means any of the following: (a) the executive officer’s willful and material breach of his or her employment agreement and the executive officer’s continued failure to cure such breach to the reasonable satisfaction of our board of directors within thirty days following written notice of such breach from our board of directors; (b) the executive officer’s conviction of, or entry of a plea of guilty or nolo contendere to a felony or a misdemeanor involving moral turpitude; (c) the executive officer’s willful commission of an act of fraud, breach of trust or dishonesty, including without limitation embezzlement or an act that results in material damage or harm to our business, financial condition or assets; (d) the executive officer’s intentional damage or destruction of substantial property of SCYNEXIS; or (e) the executive officer’s breach of the terms of his or her confidentiality agreement with us.
For purposes of these agreements, the term “good reason” means any of the following without the executive officer’s express written consent: (a) assignment to, or withdrawal from, the executive officer of any duties or responsibilities that results in a material diminution in the executive officer’s authority, duties or responsibilities as in effect immediately prior to such change; (b) a material diminution in the authority, duties or responsibilities of the supervisor to whom the executive officer is required to report, including (if applicable) a requirement that the executive officer report to a corporate officer or employee instead of reporting directly to our board of directors; (c) a material reduction by us of the executive officer’s annual base salary; (d) a relocation of the executive officer or our principal executive offices if the executive officer’s principal office is at such offices, to a location more than 60 miles from the location at which the executive officer is then performing his or her duties; or (e) a material breach by us of any provision of the executive officer’s employment agreement or any other enforceable written agreement between us and the executive officer.
Before an executive officer may terminate employment for “good reason,” the executive officer must notify us in writing, we must fail to remedy or cure the alleged “good reason” and the executive officer must then terminate employment, all within prescribed time periods.
Employment Agreements
We have entered into agreements with each of the executive officers in connection with his or her employment with us. With the oversight and approval of our board of directors, each of these employment agreements was negotiated on our behalf by our Chief Executive Officer, Dr. Ribeill, with the exception of
116
his own employment agreement. These agreements provided for “at will” employment and set forth the terms and conditions of employment of each named executive officer, including base salary, target annual bonus opportunity, standard employee benefit plan participation, initial stock option grant and vesting provisions with respect to the initial stock option grant. These employment agreements were each subject to execution of our standard confidential information and invention assignment agreement.
Employment agreement with Dr. Ribeill. We entered into an employment agreement with Dr. Ribeill in December 2001 setting forth the terms of Dr. Ribeill’s employment. Pursuant to the agreement, Dr. Ribeill was initially paid a salary of $125,000 and was eligible to receive a performance bonus based on a target amount of 30% of his base salary and certain stock options under our 2009 Plan. We entered into an amended and restated employment agreement with Dr. Ribeill in December 2012, which replaced and superseded his prior employment agreement, effective in December 2012. Pursuant to this agreement, Dr. Ribeill was initially paid an annual salary of $250,108 and was eligible to receive a performance bonus based on a target amount of 50% of his base salary, as determined by our board of directors based upon the achievement of performance objectives mutually agreed upon by Dr. Ribeill and our board of directors.
Employment agreement with Mr. Osborne. We entered into an employment agreement with Mr. Osborne in November 2003 setting forth the terms of Mr. Osborne’s employment. Pursuant to the agreement, Mr. Osborne was initially paid an annual salary of $220,000 and was eligible to receive a performance bonus based on a target amount of 30% of his base salary and certain stock options under our 2009 Plan. We entered into an amended and restated employment agreement with Mr. Osborne in December 2012, which replaced and superseded his prior employment agreement, effective in December 2012. Pursuant to this agreement, Mr. Osborne was initially paid an annual salary of $250,118 and was eligible to receive a performance bonus based on a target amount of 30% of his base salary, as determined by our board of directors based upon the achievement of performance objectives mutually agreed upon by Mr. Osborne and our board of directors.
Employment agreement with Ms. Pruette. We entered into an employment agreement with Ms. Pruette in August 2012 setting forth the terms of Ms. Pruette’s employment. Pursuant to the agreement, Ms. Pruette was initially paid an annual salary of $235,000 and was eligible to receive a performance bonus based on a target amount of 30% of her base salary, as determined by our board of directors based upon the achievement of performance objectives mutually agreed upon by Ms. Pruette and our board of directors.
Employment agreement with Dr. Sable. We entered into an employment agreement with Dr. Sable in January 2014 setting forth the terms of Dr. Sable’s employment. Pursuant to the agreement, Dr. Sable shall be paid an annual salary of $350,000 and is eligible to receive a performance bonus based on a target amount of 40% of her base salary, as determined by our board of directors based upon the achievement of performance objectives mutually agreed upon by Dr. Sable and our board of directors. In addition, pursuant to the terms of Dr. Sable’s employment agreement, in January 2014 our board of directors approved the grant of an option to Dr. Sable to purchase 951,393 shares of our common stock with an exercise price of $2.70 per share. In addition, Dr. Sable’s employment agreement provides for severance benefits as more fully described in the section Change in Control Severance Benefits above.
Employment agreement with Dr. Doelling. We entered into an offer letter with Dr. Doelling in September 2013 setting forth the terms of Dr. Doelling’s employment. Pursuant to the offer letter, Dr. Doelling was initially paid an annual salary of $170,000 and was eligible to participate in our team bonus program in addition to the company’s standard employee benefits.
117
Equity Incentive Plans
The principal features of our equity incentive plans are summarized below. These summaries are qualified in their entirety by reference to the actual text of the plans, which are filed as exhibits to the registration statement of which this prospectus is a part.
2014 Equity Incentive Plan
Our board of directors adopted the 2014 Equity Incentive Plan, or the 2014 Plan, on , and we expect our stockholders will approve the 2014 Plan prior to the closing of this offering. We expect that the 2014 Plan will become effective on the date the registration statement of which this prospectus forms a part is declared effective by the SEC. The 2014 Plan will be the successor to and continuation of our 2009 Stock Option Plan, or the 2009 Plan, which is described below. Once the 2014 Plan becomes effective, no further grants will be made under the 2009 Plan.
Stock Awards. The 2014 Plan provides for the grant of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance-based stock awards and other forms of equity compensation (collectively, stock awards), all of which may be granted to eligible employees, non-employee directors and consultants of us and our affiliates. Additionally, the 2014 Plan provides for the grant of performance cash awards. Incentive stock options may be granted only to our employees. All other awards may be granted to employees and to non-employee directors and consultants.
Share Reserve. Initially, the aggregate number of shares of our common stock that may be issued pursuant to stock awards under the 2014 Plan after the 2014 Plan becomes effective is the sum of: (1) shares; (2) the number of shares reserved for issuance under our 2009 Plan at the time the 2014 Plan becomes effective; and (3) any shares subject to outstanding stock options or other stock awards that would have otherwise returned to our 2009 Stock Option Plan (such as upon the expiration or termination of a stock option granted under such plan prior to vesting). Additionally, the number of shares of our common stock reserved for issuance under the 2014 Plan will automatically increase on January 1 of each year, beginning on January 1, 2015 (assuming the 2014 Plan becomes effective in 2014) and continuing through and including January 1, 2024, by % of the total number of shares of our capital stock outstanding on December 31 of the preceding calendar year, or a lesser number of shares as determined by our board of directors. The maximum number of shares that may be issued upon the exercise of incentive stock options under our 2014 Plan is shares.
The maximum number of shares of our common stock subject to stock awards granted during a single fiscal year to any non-employee director, taken together with any cash fees paid to such non-employee director during the fiscal year, shall not exceed $ in total value (calculating the value of any such stock awards based on the grant date fair value of such stock awards for financial reporting purposes and excluding, for this purpose, the value of any dividend equivalent payments paid pursuant to any stock award granted in a previous fiscal year).
If a stock award granted under the 2014 Plan expires or otherwise terminates without all of the shares covered by the stock award having been issued, or is settled in cash, the shares of our common stock not acquired pursuant to the stock award again will become available for subsequent issuance under the 2014 Plan. In addition, the following types of shares under the 2014 Plan may become available for the grant of new stock awards under the 2014 Plan: (1) shares that are forfeited to or repurchased by us prior to become fully vested; (2) shares withheld to satisfy income or employment withholding taxes; or (3) shares used to pay the exercise price or purchase price of a stock award. Shares issued under the 2014 Plan may be previously unissued shares or reacquired shares bought by us on the open market. As of the date hereof, no awards have been granted and no shares of our common stock have been issued under the 2014 Plan.
118
Administration. Our board of directors, or a duly authorized committee of our board of directors, has the authority to administer the 2014 Plan as the plan administrator. Our board of directors may also delegate to one or more of our officers the authority to (1) designate employees (other than other officers) to be recipients of certain stock awards and (2) determine the number of shares of common stock to be subject to such stock awards. Subject to the terms of the 2014 Plan, our board of directors or the authorized committee, referred to herein as the plan administrator, determines recipients, dates of grant, the numbers and types of stock awards to be granted and the terms and conditions of the stock awards, including the period of their exercisability and the vesting schedule applicable to a stock award. Subject to the limitations set forth below, the plan administrator will also determine the exercise price, strike price or purchase price of awards granted and the types of consideration to be paid for the award.
The plan administrator has the authority to modify outstanding awards under the 2014 Plan. Subject to the terms of our 2014 Plan, the plan administrator has the authority to reduce the exercise, purchase or strike price of any outstanding stock award, cancel any outstanding stock award in exchange for new stock awards, cash or other consideration, or take any other action that is treated as a repricing under generally accepted accounting principles, with the consent of any adversely affected participant.
Stock Options. Incentive stock options and nonstatutory stock options are granted pursuant to stock option agreements adopted by the plan administrator. The plan administrator determines the exercise price for stock options, within the terms and conditions of the 2014 Plan, provided that the exercise price of a stock option generally cannot be less than 100% of the fair market value of our common stock on the date of grant. Options granted under the 2014 Plan vest at the rate specified by the plan administrator.
The plan administrator determines the term of stock options granted under the 2014 Plan, which term may be for a maximum of 10 years. Unless the terms of the option holder’s stock option agreement provide otherwise, if an option holder’s service relationship with us, or any of our affiliates, ceases for any reason other than disability, death or cause, the option holder may generally exercise any vested options for a period of three months following the option holder’s cessation of service. The option term may be extended in the event that exercise of the option or sale of the underlying shares following such a termination of service is prohibited by applicable securities laws or by our insider trading policy. If an option holder’s service relationship with us or any of our affiliates ceases due to disability or death, or an option holder dies within a specified period following cessation of service, the option holder or a beneficiary may generally exercise any vested options for a period of twelve months in the event of disability and 18 months in the event of death. In the event of a termination for cause, options generally terminate immediately upon the termination of the individual’s service for cause. In no event may an option be exercised beyond the expiration of its term.
Acceptable consideration for the purchase of common stock issued upon the exercise of a stock option will be determined by the plan administrator and may include the following methods: (1) cash, check, bank draft or money order; (2) a broker-assisted cashless exercise procedure; (3) the tender of shares of our common stock previously owned by the option holder; (4) if the option is a nonstatutory stock option, by a net exercise arrangement; and (5) other legal consideration approved by the plan administrator and set forth in the applicable award agreement.
Unless the plan administrator provides otherwise, options generally are not transferable except by will, the laws of descent and distribution, or pursuant to a domestic relations order. An option holder may designate a beneficiary, however, who may exercise the option following the option holder’s death.
Tax Limitations on Incentive Stock Options. The aggregate fair market value, determined at the time of grant, of our common stock with respect to incentive stock options that are exercisable for the first time by an option holder during any calendar year under all of our stock plans may not exceed $100,000. Options or portions thereof that exceed such limit will generally be treated as nonstatutory stock options. No incentive
119
stock option may be granted to any person who, at the time of grant, owns or is deemed to own stock possessing more than 10% of our total combined voting power or that of any of our affiliates unless (1) the option exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant and (2) the term of the incentive stock option does not exceed five years from the date of grant.
Restricted Stock Unit Awards. Restricted stock unit awards are granted pursuant to restricted stock unit award agreements adopted by the plan administrator. Restricted stock unit awards may be granted in consideration for any form of legal consideration. A restricted stock unit award may be settled by cash, delivery of stock, a combination of cash and stock as deemed appropriate by the plan administrator, or in any other form of consideration set forth in the restricted stock unit award agreement. Additionally, dividend equivalents may be credited in respect of shares covered by a restricted stock unit award. Except as otherwise provided in the applicable award agreement, restricted stock units that have not vested will be forfeited upon the participant’s cessation of continuous service for any reason.
Restricted Stock Awards. Restricted stock awards are granted pursuant to restricted stock award agreements adopted by the plan administrator. A restricted stock award may be awarded in consideration for cash, check, bank draft or money order, past services to us, or any other form of legal consideration that may be acceptable to our board of directors and permissible under applicable law. The plan administrator determines the terms and conditions of restricted stock awards, including vesting and forfeiture restrictions. If a participant’s service relationship with us ceases for any reason, we may receive through a forfeiture condition or a repurchase right any or all of the shares of common stock held by the participant that have not vested as of the date the participant terminates service with us.
Stock Appreciation Rights. Stock appreciation rights are granted pursuant to stock appreciation grant agreements adopted by the plan administrator. The plan administrator determines the purchase price or strike price for a stock appreciation right, which generally cannot be less than 100% of the fair market value of our common stock on the date of grant. Upon the exercise of a stock appreciation right, we will pay the participant an amount equal to the product of (1) the excess, if any, of the per share fair market value of our common stock on the date of exercise over the purchase price or strike price, and (2) the number of shares of common stock with respect to which the stock appreciation right is exercised. This amount may be paid in shares of our common stock, in cash, in any combination of cash and shares of our common stock or in any other form of consideration, as determined by the plan administrator and set forth in the award agreement. A stock appreciation right granted under the 2014 Plan vests at the rate specified in the stock appreciation right agreement as determined by the plan administrator.
The plan administrator determines the term of stock appreciation rights granted under the 2014 Plan, which may be up to a maximum of 10 years. Unless the terms of a participant’s stock appreciation right agreement provides otherwise, if a participant’s service relationship with us or any of our affiliates ceases for any reason other than cause, disability or death, the participant may generally exercise any vested stock appreciation right for a period of three months following the cessation of service. The term of the stock appreciation right may be further extended in the event that exercise of the stock appreciation right following such a termination of service is prohibited by applicable securities laws or by our insider trading policy. If a participant’s service relationship with us, or any of our affiliates, ceases due to disability or death, or a participant dies within a certain period following cessation of service, the participant (or, if applicable, a beneficiary) may generally exercise any vested stock appreciation right for a period of twelve months in the event of disability and 18 months in the event of death. In the event of a termination for cause, stock appreciation rights generally terminate immediately upon the occurrence of the event giving rise to the termination of the individual’s service for cause. In no event may a stock appreciation right be exercised beyond the expiration of its term.
120
Other Stock Awards. The plan administrator may grant other awards based in whole or in part by reference to our common stock. The plan administrator will set the number of shares under the stock award and all other terms and conditions of such awards.
Changes to Capital Structure. In the event there is a specified type of change in our capital structure, such as a stock split, reverse stock split, or recapitalization, appropriate adjustments will be made to (1) the class and maximum number of shares reserved for issuance under the 2014 Plan, (2) the class and maximum number of shares by which the share reserve may increase automatically each year, (3) the class and maximum number of shares that may be issued upon the exercise of incentive stock options, (4) the class and maximum number of shares subject to stock awards that can be granted in a calendar year (as established under the 2014 Plan pursuant to Section 162(m) of the Internal Revenue Code), and (5) the class and number of shares and exercise price, strike price, or purchase price, if applicable, of all outstanding stock awards.
Corporate Transaction. Unless otherwise provided in an award agreement or any other written agreement between us and a participant, in the event of a corporate transaction, the plan administrator will take any one or more of the following actions with respect to outstanding stock awards, contingent upon the closing of the corporate transaction:
| Ÿ | arrange for the surviving corporation or acquiring corporation (or its parent) to assume or continue outstanding stock awards or substitute a similar award for such stock award; |
| Ÿ | arrange for the assignment or lapse of any reacquisition or repurchase rights; |
| Ÿ | accelerate the vesting, in whole or in part, of stock awards to a date prior to the effective time of a corporate transaction, with such stock award terminating if not exercised (if applicable) at or prior to the effective time of such corporate transaction; |
| Ÿ | cancel outstanding awards in exchange for consideration, if any, as the plan administrator determines appropriate; and |
| Ÿ | cancel or arrange for the cancellation of the stock award, to the extent not vested or not exercised prior to the effective time of a corporate transaction, in exchange for a payment, in such form as determined by the plan administrator, equal to the excess (if any) of the value of the property the participant would have received upon exercise of the stock award immediately prior to the effective time of the corporate transaction over any exercise price payable by the participant in connection with the exercise. |
The plan administrator need not take the same action or actions with respect to all stock awards or portions thereof or with respect to all participants.
Under the 2014 Plan, a corporate transaction generally occurs upon the consummation of: (1) a sale or other disposition of all or substantially all of our assets; (2) a sale or other disposition of at least 90% of our outstanding securities; (3) a merger, consolidation or similar transaction following which we are not the surviving corporation; or (4) a merger, consolidation or similar transaction following which we are the surviving corporation but the shares of our common stock outstanding immediately prior to the transaction are converted or exchanged into other property by virtue of the transaction.
Change of Control. The plan administrator may provide, in an individual award agreement or in any other written agreement between a participant and us that the stock award will be subject to additional acceleration of vesting and exercisability in the event of a change of control. Under the 2014 Plan, a change of control generally occurs upon: (1) the acquisition by a person or entity of more than 50% of our combined voting power, other than by merger, consolidation or similar transaction (and excluding the acquisition of our
121
securities by certain individuals or affiliates, as set forth in the 2014 Plan); (2) a consummated merger, consolidation or similar transaction immediately after which our stockholders cease to own more than 50% of the combined voting power of the surviving entity; (3) a consummated sale, lease, exclusive license or other disposition of all or substantially all of our consolidated assets; or (4) individuals who constitute our incumbent board of directors cease to constitute at least a majority of our board of directors.
Amendment and Termination. Our board of directors generally has the authority to amend, suspend or terminate our 2014 Plan at any time, provided that except in specified circumstances, no such action may be taken without such participant’s written consent if it would materially impair the existing rights of any participant. No incentive stock options may be granted after the tenth anniversary of the date on which our board of directors adopted our 2014 Plan.
2009 Stock Option Plan
Our board of directors adopted the 2009 Stock Option Plan, or the 2009 Plan, on October 22, 2009. Our 2009 Plan provides for the grant of incentive stock options to our employees, and for the grant of nonstatutory stock options to our employees, directors and consultants.
Our 2014 Plan, which is described above, will become effective on the date the registration statement of which this prospectus forms a part is declared effective by the SEC. We will not grant any additional options under our 2009 Plan following the date on which the 2014 Plan becomes effective. However, any outstanding options granted under the 2009 Plan will remain outstanding, subject to the terms of our 2009 Plan, and the applicable stock option agreements, until such outstanding options are exercised or until they terminate or expire by their terms.
Authorized Shares. As of , 2014, the maximum number of shares of our common stock that may be issued under our 2009 Plan is shares, which includes (1) shares of our common stock issuable upon the exercise of outstanding options, (2) shares of our common stock that are issuable upon the exercise of outstanding options under the 1999 Plan that may become available for grant under the 2009 Plan upon termination, surrender or cancellation without having been exercised in full, and (3) shares of our common stock reserved for further issuance under the 2009 Plan.
Plan Administration. Our board of directors or a duly authorized committee of our board of directors administers our 2009 Plan. Subject to the terms of our 2009 Plan, the plan administrator has the authority to select the employees, directors and consultants to whom options may be granted, determine the terms of the options (including the vesting schedule), the number of shares of common stock subject to options, the exercise price, the form of consideration payable upon exercise of the options, and the terms of the award agreements for use under our 2009 Plan. Our board of directors may, at any time, provide that any option will become immediately exercisable in full or in part. In addition, our board of directors may, without stockholder approval, (1) amend any outstanding option granted to provide an exercise price per share that is lower than the then-current exercise price of the outstanding option (provided that the amended exercise price is at least equal to the then-current fair market value) and (2) cancel any outstanding option and grant in substitution new options covering the same or a different number of shares of our common stock and having an exercise price per share lower than the then-current exercise price per share of the cancelled option.
Stock Options. Each option is evidenced by an option award agreement and must be granted with an exercise price at least equal to 100% of the fair market value of our common stock on the date the option is granted (or at least 110% of the fair market value if the option is an incentive stock option granted to a participant who owns more than 10% of the total combined voting power of all classes of our outstanding stock, or a ten percent stockholder). Incentive stock options granted to ten percent stockholders may not have a term greater than five years.
122
Options may be exercised at such times and subject to such terms and conditions as specified in the applicable option agreement. The exercise price of an option may be paid as follows: (1) in cash or by check; (2) to the extent approved by our board of directors, in its sole discretion, provided our shares are registered under the Exchange Act through a broker-assisted exercise procedure; (3) by delivery of shares of our common stock previously owned by the participant; (4) to the extent approved by our board of directors, by delivery of a promissory note or by payment of other lawful consideration; or (5) by any combination of the above permitted forms of payment.
A participant must satisfy all applicable federal, state and local or other income and employment tax withholding obligations before we will deliver stock certificates or otherwise recognize ownership of our common stock under an option. If provided for in an option or approved by our board of directors, a participant may satisfy any tax withholding obligations in whole or in part by delivery of shares of our common stock, including shares retained from an option creating the tax obligation.
Termination of Service. Our board of directors will determine the effect on an option of the disability, death, termination of employment, authorized leave of absence or other change in the employment or other status of a participant and the extent to which, and the period during which, the participant (or the participant’s legal representative) may exercise rights under the option following any such change in employment or status.
Capitalization Adjustments. In the event of any stock split, reverse stock split, stock dividend, recapitalization, combination of shares, or other similar change in capitalization or event, or any dividend or distributions to holders of our common stock other than an ordinary cash dividend, our board of directors will equitably adjust (1) the number and class of securities available under the 2009 Plan, and (2) the number and class of securities and the exercise price per share of each outstanding option.
Change in Control. In the event of a change in control, any then unexercisable portion of an outstanding option will become immediately exercisable as of a date prior to, but conditioned upon, the change in control, determined by our board of directors, except to the extent that (1) the option is either to be assumed by, or substituted with a comparable option to purchase shares of, the successor corporation (or parent thereof), (2) the option is to be replaced with a cash incentive program of the successor corporation which preserves the spread existing on the unvested option at the time of the change in control and provides for subsequent payout in accordance with the same vesting schedule applicable to the option, or (3) the acceleration of the option is subject to other limitations imposed by our board of directors at the time the option was granted. Our board of directors may provide that any options which become exercisable solely by reason of these provisions and remain unexercised will terminate effective as of the date of the change in control. For purposes of the 2009 Plan, a change in control will be deemed to have occurred upon the consummation of a merger, consolidation, corporate reorganization, or sale or transfer of substantially all of our assets or stock (other than a reincorporation transaction or one in which the holders of our capital stock immediately prior to the merger or consolidation continue to hold at least a majority of the voting power of the surviving corporation).
Transferability. Unless otherwise provided by our board of directors, options may not be sold, assigned, transferred, pledged or otherwise encumbered by the person to whom they are granted, either voluntarily or by operation of law, except by will or the laws of descent and distribution or, other than in the case of an incentive stock option, pursuant to a qualified domestic relations order. During the life of a participant, an option will be exercisable only by the participant.
Amendment and Termination. Our board of directors may amend, modify or terminate any outstanding option, provided that no such action may materially and adversely affect the participant without such participant’s consent. No options may be granted under the 2009 Plan after the expiration of 10 years from the
123
earlier of: (1) the date on which the 2009 Plan was adopted by our board of directors; and (2) the date on which the 2009 Plan was approved by our stockholders. Our board of directors generally may amend, suspend or terminate the 2009 Plan or any portion thereof at any time; provided, that to the extent that any amendment requires stockholder approval, the 2009 Plan may not be so amended without such approval.
1999 Stock Option Plan
Our board of directors adopted the Stock Option Plan, or the 1999 Plan, on November 4, 1999. The 1999 Plan was last amended by our board of directors on April 23, 2009 and approved by our stockholders on May 28, 2009. Our 1999 Plan provides for the grant of incentive stock options to our employees, and for the grant of nonstatutory stock options to our employees, directors and consultants.
Our 1999 Plan expired by its terms in November 2009, and we have not granted any options under our 1999 Plan since such date. However, outstanding options granted under the 1999 Plan remain subject to the terms of our 1999 Plan until such options are exercised or until they terminate or expire by their terms.
Authorized Shares. As of , there were shares of our common stock issuable upon the exercise of outstanding options under our 1999 Plan.
Plan Administration. Our board of directors or a duly authorized committee of our board of directors administers outstanding options granted our 1999 Plan.
Stock Options. The 1999 Plan authorized the grant of incentive stock options to eligible employees and nonstatutory stock options to eligible employees, directors and consultants. Each option was evidenced by an option award agreement and was granted with an exercise price determined by our board of directors, which, for incentive stock options, was required to be at least 100% of the fair market value of our common stock on the date the option was granted (or at least 110% of the fair market value, if granted to a participant who owned more than 10% of the total combined voting power of all classes of our outstanding stock, or a ten percent stockholder). The term of any option granted under the 1999 Plan was established by our board of directors, except that no incentive stock option was granted with a term greater than ten years after the date of grant (or five years, if granted to a ten percent stockholder). Payment of the exercise price may be made in cash, by check, cash equivalent or in any other form as may be permitted by our board of directors.
Termination of Service. An option will terminate and cease to be exercisable no later than three months after the date on which an option holder terminates employment or service with us, except that if an option holder’s employment or service terminates due to death (including, if the option holder dies within three months following the option holder’s termination of employment) or disability, then such option will terminate and cease to be exercisable no later than twelve months from the date of death or disability. Notwithstanding the foregoing, no incentive stock option may be exercised after the date the option holder’s employment with us is terminated for cause (as determined in the sole discretion of our board of directors).
Capitalization Adjustments. In the event of a stock dividend, stock split, reverse stock split, combination, reclassification or like change in our capital structure, our board of directors will make appropriate adjustments in the number and class of shares of stock subject to the 1999 Plan and to any outstanding options and the exercise price of any outstanding options.
Transfer of Control. In the event of a transfer of control, any then unexercisable portion of an outstanding option will become immediately exercisable as of a date prior to, but conditioned upon, the transfer of control, determined by our board of directors, except to the extent that (1) the option is either to be assumed by, or substituted with a comparable option to purchase shares of, the successor corporation (or parent thereof), (2) the option is to be replaced with a cash incentive program of the successor corporation which preserves the spread existing on the unvested option at the time of the transfer of control and provides for subsequent payout in
124
accordance with the same vesting schedule applicable to the option, or (3) the acceleration of the option is subject to other limitations imposed by our board of directors at the time the option was granted. Our board of directors may provide that any options which become exercisable solely by reason of these provisions and remain unexercised will terminate effective as of the date of the transfer of control. For purposes of the 1999 Plan, a transfer of control means a merger, consolidation, corporate reorganization, or sale or transfer of substantially all of our assets or stock (other than a reincorporation transaction or one in which the holders of our capital stock immediately prior to the merger or consolidation continue to hold at least a majority of the voting power of the surviving corporation).
Transferability. No option may be assignable or transferable by an option holder, except by will or by the laws of descent and distribution. During the lifetime of an option holder, an option will be exercisable only by the option holder.
Amendment: Termination. Our board of directors has the authority to amend the terms of an option at any time; provided, that no amendment may adversely affect any then-outstanding option or any unexercised portion of an option without the consent of the option holder (unless the amendment is required to enable an option designated as incentive stock option to so qualify).
2014 Employee Stock Purchase Plan
Our board of directors adopted the 2014 Employee Stock Purchase Plan, or ESPP, on , 2014, and we expect our stockholders to approve the ESPP prior to the closing of this offering. The purpose of the ESPP is to secure the services of new employees, to retain the services of existing employees and to provide incentives for such individuals to exert maximum efforts toward our success and that of our affiliates. The ESPP is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Internal Revenue Code.
Share Reserve. Following this offering, the ESPP authorizes the issuance of shares of our common stock pursuant to purchase rights granted to our employees or to employees of any of our designated affiliates. The number of shares of our common stock reserved for issuance will automatically increase on January 1 of each calendar year, from January 1, 2015 (assuming the ESPP becomes effective in 2014) through January 1, 2024, by the least of (1) 0.8% of the total number of shares of our common stock outstanding on December 31 of the preceding calendar year, and (2) shares; provided, that prior to the date of any such increase, our board of directors may determine that such increase will be less than the amount set forth in clauses (1) and (2). As of the date hereof, no shares of our common stock have been purchased under the ESPP.
Administration. Our board of directors has the authority administer the ESPP. The ESPP is implemented through a series of offerings under which eligible employees are granted purchase rights to purchase shares of our common stock on specified dates during such offerings. Under the ESPP, we may specify offerings with durations of not more than 27 months, and may specify shorter purchase periods within each offering. Each offering will have one or more purchase dates on which shares of our common stock will be purchased for employees participating in the offering. We currently intend to have twenty-four month offerings with four purchase periods (of approximately six months in duration) per offering, except that the first purchase period under our first offering may be shorter or longer than six months, depending on the date on which the underwriting agreement relating to this offering becomes effective. An offering under the ESPP may be terminated under specified circumstances.
Payroll Deductions. Generally, all regular employees, including executive officers, employed by us or by any of our designated affiliates, may participate in the ESPP and may contribute, normally through payroll deductions, up to 15% of their earnings (as defined in the ESPP) for the purchase of our common
125
stock under the ESPP. Unless otherwise determined by our board of directors, common stock will be purchased for the accounts of employees participating in the ESPP at a price per share equal to the lower of (a) 85% of the fair market value of a share of our common stock on the first date of an offering or (b) 85% of the fair market value of a share of our common stock on the date of purchase. For the initial offering, which we expect will commence upon the execution and delivery of the underwriting agreement relating to this offering, the fair market value on the first day of the offering period will be the price at which shares are first sold to the public.
Limitations. Employees may have to satisfy one or more of the following service requirements before participating in the ESPP, as determined by our board of directors, including: (1) being customarily employed for more than 20 hours per week; (2) being customarily employed for more than five months per calendar year; or (3) continuous employment with us or one of our affiliates for a period of time (not to exceed two years). No employee may purchase shares under the ESPP at a rate in excess of $25,000 worth of our common stock based on the fair market value per share of our common stock at the beginning of an offering for each year a purchase right is outstanding. During any purchase period, the maximum number of shares an employee may purchase on a purchase date is shares and no more than shares may be purchased by all employees on a purchase date. Finally, no employee will be eligible for the grant of any purchase rights under the ESPP if immediately after the rights are granted, the employee has voting power over 5% or more of our outstanding capital stock measured by vote or value pursuant to Section 424(d) of the Internal Revenue Code.
Changes to Capital Structure. In the event that there occurs a change in our capital structure through actions such as a stock split, merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, large nonrecurring cash dividend, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or similar transaction, the board of directors will make appropriate adjustments to (1) the number of shares reserved under the ESPP, (2) the maximum number of shares by which the share reserve may increase automatically each year, (3) the number of shares and purchase price of all outstanding purchase rights, and (4) the number of shares that are subject to purchase limits under ongoing offerings.
Corporate Transactions. In the event of certain significant corporate transactions, including: (1) a sale of all or substantially all of our assets; (2) the sale or disposition of 90% of our outstanding securities; (3) the consummation of a merger or consolidation where we do not survive the transaction; and (4) the consummation of a merger or consolidation where we do survive the transaction but the shares of our common stock outstanding immediately prior to such transaction are converted or exchanged into other property by virtue of the transaction, any then-outstanding rights to purchase our stock under the ESPP may be assumed, continued or substituted for by any surviving or acquiring entity (or its parent company). If the surviving or acquiring entity (or its parent company) elects not to assume, continue or substitute for the purchase right, then the participants’ accumulated payroll contributions will be used to purchase shares of our common stock within 10 business days prior to the corporate transaction, and the purchase rights will terminate immediately.
ESPP Amendment; Termination. Our board of directors has the authority to amend or terminate our ESPP, provided that except in certain circumstances any such amendment or termination may not materially impair any outstanding purchase rights without the holder’s consent. We will obtain stockholder approval of any amendment to our ESPP as required by applicable law or listing requirements.
401(k) Plan
We maintain a tax-qualified retirement plan that provides eligible U.S. employees with an opportunity to save for retirement on a tax advantaged basis. Eligible employees are able to defer eligible compensation
126
subject to applicable annual Internal Revenue Code limits. We have the ability to make discretionary contributions to the 401(k) plan and currently provide a $0.50 match for every dollar our employees elect to defer up to 6% of their eligible compensation. Employees’ pre-tax contributions are allocated to each participant’s individual account and are then invested in selected investment alternatives according to the participants’ directions. Employees are immediately and fully vested in their contributions, and matching contributions made by us vest in four equal annual installments over a period of four years. The 401(k) plan is intended to be qualified under Section 401(a) of the Internal Revenue Code with the 401(k) plan’s related trust intended to be tax exempt under Section 501(a) of the Internal Revenue Code. As a tax-qualified retirement plan, contributions to the 401(k) plan and earnings on those contributions are not taxable to the employees until distributed from the 401(k) plan.
Limitation on Liability and Indemnification Matters
Upon the closing of this offering, our amended and restated certificate of incorporation will contain provisions that limit the liability of our current and former directors for monetary damages to the fullest extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for any breach of fiduciary duties as directors, except liability for:
| Ÿ | any breach of the director’s duty of loyalty to us or our stockholders; |
| Ÿ | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| Ÿ | unlawful payments of dividends or unlawful stock repurchases or redemptions; or |
| Ÿ | any transaction from which the director derived an improper personal benefit. |
Such limitation of liability does not apply to liabilities arising under federal securities laws and does not affect the availability of equitable remedies, such as injunctive relief or rescission.
Our amended and restated certificate of incorporation and our amended and restated bylaws that will become effective immediately upon completion of this offering will provide that we are required to indemnify our directors and executive officers to the fullest extent permitted by Delaware law. Our amended and restated bylaws will also provide that, upon satisfaction of certain conditions, we shall advance expenses incurred by a director or executive officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of Delaware law. Our amended and restated certificate of incorporation and amended and restated bylaws will also provide our board of directors with discretion to indemnify our other officers and employees when determined appropriate by our board of directors. We have entered and expect to continue to enter into agreements to indemnify our directors and executive officers. With certain exceptions, these agreements provide for indemnification for related expenses including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. We also maintain customary directors’ and officers’ liability insurance.
The limitation on liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the
127
costs of settlement and damage awards against directors and officers as required by these indemnification provisions. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought and we are not aware of any threatened litigation that may result in claims for indemnification.
128
TRANSACTIONS WITH RELATED PERSONS
Other than compensation arrangements, we describe below transactions and series of similar transactions, since January 1, 2011, to which we were a party or will be a party, in which:
| Ÿ | the amounts involved exceeded or will exceed $120,000; and |
| Ÿ | any of our directors, executive officers, holders of more than 5% of our capital stock, or any affiliate of our directors, executive officers and holders of more than 5% of our capital stock, had or will have a direct or indirect material interest. |
Compensation arrangements for our directors and named executive officers are described elsewhere in this prospectus.
Series D-2 Preferred Stock Financing
In December 2013, we sold 1,785,712 shares of our Series D-2 preferred stock and warrants exercisable for 1,785,712 shares of our common stock to five of our existing investors for aggregate proceeds of $2.5 million, which we refer to as our 2013 financing, as follows.
| Purchasers(1) | Shares Purchased |
Warrant Shares |
Aggregate Purchase Price |
|||||||||
| Alta BioPharma Partners II, LP(2) |
1,205,648 | 1,205,648 | $ | 1,687,907.20 | ||||||||
| Alta Embarcadero BioPharma Partners II, LLC(2) |
44,352 | 44,352 | 62,092.80 | |||||||||
| F.C.P.R. Genavent |
71,428 | 71,428 | 99,999.20 | |||||||||
| FCPR Biotechnology Fund(3) |
107,142 | 107,142 | 149,998.80 | |||||||||
| Ventech Capital II(4) |
357,142 | 357,142 | 499,998.80 | |||||||||
| (1) | See “Principal Stockholders” for more information about these directors, executive officers, holders of more than 5% of our capital stock, and their affiliates. |
| (2) | Entities affiliated with Alta BioPharma Partners II, LP (“ABP II”) and Alta Embarcadero BioPharma Partners II, LLC (“AEBP II”) are holders of more than 5% of our capital stock. Dr. Penhoet, a member of our board of directors, is a director of Alta BioPharma Management II, LLC, the general partner of ABP II and manager of AEBP II. |
| (3) | FCPR Biotechnology Fund is a holder of more than 5% of our capital stock. Dr. Nothias, a member of our board of directors, is a member of the investment board of FCPR Biotechnology Fund. |
| (4) | Ventech Capital II is a holder of more than 5% of our capital stock. Dr. Chaoui, a member of our board of directors, is a venture partner of Ventech Capital II. |
129
In December 2013, we issued 6,054,255 shares of Series D-1 preferred stock and 3,956,985 shares of Series D-2 preferred stock in connection with the conversion of all outstanding principal and interest on the convertible promissory notes previously issued in our 2011 bridge financing and 2013 bridge financing, each as described below. In addition, pursuant to the terms of our 2011 bridge financing and 2013 bridge financing, we issued warrants exercisable for 1,634,792 shares and 1,815,385 shares of our common stock, respectively, with an exercise price of $0.01 per share. The following table sets forth the aggregate number of shares our Series D-1 preferred stock, Series D-2 preferred stock and warrants exercisable for shares of our common stock issued to our directors, executive officers, holders of more than 5% of our capital stock, and their affiliates in connection with the conversion of all outstanding principal and interest on the convertible promissory notes issued in our 2011 bridge financing and our 2013 bridge financing, as follows:
| Purchasers(1) | Series D-1 Shares |
Series D-2 Shares |
Warrant Shares |
|||||||||
| Alta BioPharma Partners II, LP(2) |
1,024,876 | 211,667 | 662,147 | |||||||||
| Alta Embarcadero BioPharma Partners II, LLC(2) |
37,702 | 9,563 | 24,667 | |||||||||
| Burrill Biotechnology Capital Fund(3) |
885,481 | 94,712 | 171,428 | |||||||||
| F.C.P.R. Genavent |
955,215 | 270,028 | 214,284 | |||||||||
| FCPR Biotechnology Fund(4) |
863,672 | 516,738 | 637,533 | |||||||||
| Ventech Capital II(5) |
809,584 | 2,653,665 | 1,105,868 | |||||||||
| S.R. One, Limited |
762,944 | 185,570 | 502,053 | |||||||||
| (1) | See “Principal Stockholders” for more information about these directors, executive officers, holders of more than 5% of our capital stock, and their affiliates. |
| (2) | Entities affiliated with Alta BioPharma Partners II, LP (“ABP II”) and Alta Embarcadero BioPharma Partners II, LLC (“AEBP II”) are holders of more than 5% of our capital stock. Dr. Penhoet, a member of our board of directors, is a director of Alta BioPharma Management II, LLC, the general partner of ABPII and manager of AEBP II. |
| (3) | Burrill Biotechnology Capital Fund, L.P. is a holder of more than 5% of our capital stock. Dr. Hanham, a member of our board of directors, is a former Managing Director and General Partner with Burrill & Company, an affiliate of Burrill Biotechnology Capital Fund, L.P. |
| (4) | FCPR Biotechnology Fund is a holder of more than 5% of our capital stock. Dr. Nothias, a member of our board of directors, is a member of the investment board of FCPR Biotechnology Fund. |
| (5) | Ventech Capital II is a holder of more than 5% of our capital stock. Dr. Chaoui, a member of our board of directors, is a venture partner of Ventech Capital II. |
130
Convertible Note and Warrant Issuances
In December 2011, January 2012 and May 2012, we collectively issued and sold (a) an aggregate principal amount of $11.4 million of convertible promissory notes and (b) warrants to purchase an aggregate of 530,719 shares of our common stock with an exercise price of $0.01 per share for aggregate proceeds of $5,722, to eleven investors, which we refer to as our 2011 bridge financing. In connection with our 2013 financing, these warrants were adjusted to be exercisable for an aggregate of 1,634,792 shares of our common stock with no additional proceeds to us. The following table sets forth the aggregate principal amount of such convertible promissory notes and warrants exercisable for shares of our common stock issued to our directors, executive officers, holders of more than 5% of our capital stock, and their affiliates:
| Purchasers(1) |
Principal Amount of Notes |
Initial Warrant Shares |
Warrant
Shares After Adjustment |
|||||||||
| Alta BioPharma Partners II, LLC(2) |
$ | 1,300,000 | 60,290 | 185,714 | ||||||||
| Alta Embarcadero BioPharma Partners II, LLC(2) |
50,000 | 2,319 | 7,142 | |||||||||
| Burrill Biotechnology Capital Fund, L.P.(3) |
1,200,000 | 55,652 | 171,428 | |||||||||
| F.C.P.R. Genavent |
1,500,000 | 69,565 | 214,284 | |||||||||
| FCPR Biotechnology Fund(4) |
1,500,000 | 69,565 | 214,284 | |||||||||
| Ventech Capital II(5) |
4,000,000 | 185,507 | 571,428 | |||||||||
| S.R. One, Limited |
1,000,000 | 46,385 | 142,856 | |||||||||
| (1) | See “Principal Stockholders” for more information about these directors, executive officers, holders of more than 5% of our capital stock, and their affiliates. |
| (2) | Entities affiliated with Alta BioPharma Partners II, LLC (“ABP II”) and Alta Embarcadero BioPharma Partners II, LLC (“AEBP II”) are holders of more than 5% of our capital stock. Dr. Penhoet, a member of our board of directors, is a managing director of Alta BioPharma Management II, LLC, the general partner of ABPII and manager of AEBP II. |
| (3) | Burrill Biotechnology Capital Fund, L.P. is a holder of more than 5% of our capital stock. Dr. Hanham, a member of our board of directors, is a former Managing Director and General Partner with Burrill & Company, an affiliate of Burrill Biotechnology Capital Fund, L.P. |
| (4) | FCPR Biotechnology Fund is a holder of more than 5% of our capital stock. Dr. Nothias, a member of our board of directors, is a member of the investment board of FCPR Biotechnology Fund. |
| (5) | Ventech Capital II is a holder of more than 5% of our capital stock. Dr. Chaoui, a member of our board |
of directors, is a venture partner of Ventech Capital II.
In June 2013, we issued and sold an aggregate principal amount of $899,053 of convertible promissory notes to six investors, which we refer to as our 2013 bridge financing. The following table sets forth the aggregate principal amount of such convertible promissory notes and warrants issued on December 11, 2013, pursuant to the 2013 bridge financing and exercisable for shares of our common stock issued to our directors, executive officers, holders of more than 5% of our capital stock, and their affiliates:
| Purchaser(1) |
Principal Amount of Notes |
Warrant Shares |
||||||
| Alta BioPharma Partners II, LP(2) |
$ | 235,949 | 476,333 | |||||
| Alta Embarcadero BioPharma Partners II, LLC(2) |
8,680 | 17,525 | ||||||
| FCPR Biotechnology Fund(3) |
209,609 | 423,249 | ||||||
| Ventech Capital II(4) |
264,676 | 534,440 | ||||||
| S.R. One, Limited |
177,889 | 359,197 | ||||||
131
| (1) | See “Principal Stockholders” for more information about these directors, executive officers, holders of more than 5% of our capital stock, and their affiliates. |
| (2) | Entities affiliated with Alta BioPharma Partners II, LP (“ABP II”) and Alta Embarcadero BioPharma Partners II, LLC (“AEBP II”) are holders of more than 5% of our capital stock. Dr. Penhoet, a member of our board of directors, is a director of Alta BioPharma Management II, LLC, the general partner of ABP II and manager of AEBP II. |
| (3) | FCPR Biotechnology Fund is a holder of more than 5% of our capital stock. Dr. Nothias, a member of our board of directors, is a member of the investment board of FCPR Biotechnology Fund. |
| (4) | Ventech Capital II is a holder of more than 5% of our capital stock. Dr. Chaoui, a member of our board of directors, is a venture partner of Ventech Capital II. |
Investor Rights Agreement
We are party to an investor rights agreement that provides holders of our convertible preferred stock and shares of our common stock into which those shares will be converted at the closing of this offering, including certain holders of 5% of our capital stock and entities affiliated with certain of our directors, with certain registration rights, including the right to demand that we file a registration statement or request that their shares be covered by a registration statement that we are otherwise filing. The investor rights agreement also provides for a right of first refusal in favor of certain holders of our stock with regard to certain issuances of our capital stock. The rights of first refusal will not apply to, and will terminate upon, closing of this offering. For a more detailed description of these registration rights, see the section of this prospectus titled “Description of Capital Stock—Registration Rights.”
Voting Agreement
We are party to a voting agreement under which certain holders of our capital stock, including certain holders of 5% of our capital stock and entities affiliated with certain of our directors, have agreed to vote in a certain way on certain matters, including with respect to the election of directors. Upon the closing of this offering, the voting agreement will terminate and none of our stockholders will have any special rights regarding the election or designation of members of our board of directors.
Right of First Refusal and Co-Sale Agreement
We are party to a right of first refusal and co-sale agreement with holders of our convertible preferred stock and our founders, including certain holders of 5% of our capital stock and entities affiliated with certain of our directors, pursuant to which the holders of convertible preferred stock have a right of first refusal and co-sale in respect of certain sales of securities by our founders. Upon the closing of this offering, the right of first refusal and co-sale agreement will terminate.
Indemnification Agreements
Our amended and restated certificate of incorporation, which will be effective upon the closing of this offering, will contain provisions limiting the liability of directors, and our amended and restated bylaws will provide that we will indemnify each of our directors and executive officers to the fullest extent permitted under Delaware law. Our amended and restated certificate of incorporation and amended and restated bylaws will also provide our board of directors with discretion to indemnify our other officers and employees when determined appropriate by our board of directors. In addition, we have entered and expect to continue to enter into agreements to indemnify our directors and executive officers. For more information regarding these agreements, see the section of this prospectus titled “Executive Compensation—Limitations on Liability and Indemnification Matters.”
132
Change in Control Arrangements
We have entered into employment agreements with each of our executive officers that provide certain change in control severance benefits, as described in greater detail in the section of this prospectus titled “Executive Compensation—Change in Control Severance Benefits.”
Loan Guarantee with Sanofi
Sanofi-Aventis S.A., or Sanofi, is an affiliate of Merial, a holder of more than 5% of our capital stock. In connection with our 2010 Credit Agreement with HSBC Bank USA, National Association, in April 2010, we entered into a Stand Alone First Demand Guarantee, which we refer to as the Sanofi Guarantee, and a Reimbursement and General Security Agreement, which we refer to as the Sanofi Reimbursement Agreement, with Sanofi, both of which were amended in March 2013. The Sanofi Guarantee provides that Sanofi has agreed to guarantee our loan obligations under the 2010 Credit Agreement, and the Sanofi Reimbursement Agreement provides that we will reimburse Sanofi for any payment it makes to the lender under the Sanofi Guarantee. In connection with the Sanofi Reimbursement Agreement, we also entered into a side letter in April 2010, which provides that we will either (1) subject to the prior written request of Sanofi, apply the net proceeds of certain capital-raising activities to repay all amounts owed under our 2010 Credit Agreement to fully release Sanofi from its obligations under the Sanofi Guarantee, or (2) provide Sanofi with a waiver from HSBC Bank USA, National Association fully releasing Sanofi from its obligations under the Sanofi Guarantee. The amendments to the Sanofi Guarantee and Sanofi Reimbursement Agreement entered into in March 2013 provide that the terms of these agreements extend until January 30, 2015.
In April 2010, we also entered into a Right of First Negotiation Agreement with Sanofi, which granted Sanofi an exclusive right of first negotiation with respect to intellectual property rights related to SCY-635. This agreement expired on April 9, 2012.
In March 2013, we entered into a Board Observation Rights Agreement with Sanofi, which provides Sanofi with the right to designate one observer to attend meetings of our board of directors.
Research Services Agreement with Merial
We entered into a Research Services Agreement with Merial effective in January 2012, under which we perform research services for Merial, including the synthesis, purification, and characterization of individual or libraries of compounds, phenotypic screening of compounds, and further testing and optimizing of compounds for the use of commercializing animal health products. This agreement expires on December 31, 2014. See “Business—Collaborations and Licensing Agreements” for more information.
Engagement Letters with Burrill Securities
In March 2013, we entered into an engagement letter with Burrill Securities, an affiliate of Burrill Biotechnology Capital Fund, L.P., a holder of more than 5% of our capital stock, and an entity with which one of our directors, Dr. Hanham, was affiliated at the time. Pursuant to the letter, we engaged Burrill Securities to assist us with the identification of certain strategic alternatives. Under the letter, we would have owed Burrill Securities a success fee of $1.0 million upon the closing of specified strategic transactions during the term of the letter or within twelve months after the end of the term of the letter. The term of the letter expired on September 6, 2013.
In May 2013, we entered into an engagement letter with Burrill Securities. Pursuant to the letter, we engaged Burrill Securities to assist us with the identification of certain strategic alternatives. Under the letter, we would have owed Burrill Securities a success fee of 5% of the transaction value of any strategic transaction or financing transaction resulting from the engagement and closed during the term of the letter or within twelve months after the end of the term of the letter. The term of the letter expired on November 17, 2013.
133
Policies and Procedures for Related Person Transactions
Our board of directors adopted a policy that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock and any members of the immediate family of any of the foregoing persons are not permitted to enter into a related person transaction with us without the prior consent of our audit committee. Any request for us to enter into a transaction with an executive officer, director, nominee for election as a director, beneficial owner of more than 5% of any class of our common stock or any member of the immediate family of any of the foregoing persons in which the amount involved exceeds $120,000 and such person would have a direct or indirect interest must first be presented to our audit committee for review, consideration and approval. In approving or rejecting any such proposal, our audit committee is to consider the material facts of the transaction, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances and the extent of the related person’s interest in the transaction. We did not have a formal review and approval policy for related party transactions at the time of any of the transactions described above. However, all of the transactions described above were entered into after presentation, consideration and approval by our board of directors.
134
The following table sets forth information regarding the beneficial ownership of our common stock as of December 11, 2013, by the following:
| Ÿ | each of our directors and named executive officers; |
| Ÿ | all of our directors and executive officers as a group; and |
| Ÿ | each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common stock. |
Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, including options and warrants that are currently exercisable or exercisable within 60 days of December 11, 2013. Shares of our common stock issuable pursuant to stock options and warrants are deemed outstanding for computing the percentage of the person holding such options or warrants and the percentage of any group of which the person is a member but are not deemed outstanding for computing the percentage of any other person. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons named in the table below have sole voting and investment power with respect to all shares of common stock shown that they beneficially own, subject to community property laws where applicable. The information does not necessarily indicate beneficial ownership for any other purpose, including for purposes of Section 13(d) and 13(g) of the Securities Exchange Act of 1934, as amended.
Our calculation of the percentage of beneficial ownership prior to this offering is based on 40,720,182 shares of common stock outstanding as of December 11, 2013, assuming the conversion of all outstanding shares of our convertible preferred stock into common stock immediately upon the closing of this offering, as if this conversion had occurred as of December 11, 2013. Our calculation of the percentage of beneficial ownership after this offering is based on shares of common stock outstanding immediately after the closing of this offering (assuming no exercise of the underwriters’ over-allotment option to purchase additional shares of our common stock).
Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o SCYNEXIS, Inc., 3501 C Tricenter Boulevard, Durham, North Carolina 27713.
| Percentage of Shares Beneficially Owned | ||||||||||
| Name of Beneficial Owner |
Number of Shares Beneficially Owned |
Before the Offering |
After the Offering | |||||||
| 5% Stockholders: |
||||||||||
| Alta BioPharma Partners II, LP and affiliate(1) |
7,848,245 | 18.40 | % | |||||||
| Burrill Biotechnology Capital Fund, L.P.(2) |
3,966,303 | 9.70 | % | |||||||
| F.C.P.R. Genavent(3) |
4,614,627 | 11.25 | % | |||||||
| FCPR Biotechnology Fund(4) |
4,973,967 | 12.00 | % | |||||||
| Merial Limited(5) |
2,583,511 | 6.34 | % | |||||||
| S.R. One, Limited(6) |
3,910,476 | 9.49 | % | |||||||
| Ventech Capital II(7) |
7,854,442 | 18.62 | % | |||||||
135
| Percentage of Shares Beneficially Owned | ||||||||||
| Name of Beneficial Owner |
Number of Shares Beneficially Owned |
Before the Offering |
After the Offering | |||||||
| Named Executive Officers and Directors: |
||||||||||
| Yves J. Ribeill, Ph.D.(8) |
955,301 | 2.31 | % | |||||||
| Eileen C. Pruette(9) |
45,800 | * | ||||||||
| Charles F. Osborne, Jr.(10) |
352,963 | * | ||||||||
| Vivian W. Doelling, Ph.D. |
— | * | ||||||||
| Pamela J. Kirby, Ph.D.(11) |
273,000 | * | ||||||||
| Laurent Arthaud(12) |
167,033 | * | ||||||||
| Mounia Chaoui, Ph.D.(13) |
7,854,442 | 18.62 | % | |||||||
| Ann F. Hanham, Ph.D.(14) |
3,966,303 | 9.70 | % | |||||||
| Patrick J. Langlois, Ph.D.(15) |
175,000 | * | ||||||||
| Jean-Yves Nothias, Ph.D.(16) |
4,973,967 | 12.00 | % | |||||||
| Edward E. Penhoet, Ph.D. |
— | — | ||||||||
| All executive officers and directors as a group (11 persons)(17) |
18,763,809 | 45.11 | % | |||||||
| * | Less than 1% of the outstanding shares of common stock |
| (1) | Consists of shares issuable upon conversion of 570,159 shares of Series C preferred stock, 1,024,876 shares of Series D-1 preferred stock, 1,417,315 shares of Series D-2 preferred stock, and 1,867,795 shares of common stock issuable pursuant to warrants exercisable within 60 days of December 11, 2013 held by Alta BioPharma Partners II, LP and shares issuable upon conversion of 20,975 shares of Series C preferred stock, 37,702 shares of Series D-1 preferred stock, 53,915 shares of Series D-2 preferred stock and 69,019 shares of common stock held by Alta Embarcadero BioPharma Partners II, LLC. Alta Partners II, Inc. provides investment advisory services to several venture capital funds, including Alta BioPharma Partners II, L.P. (“ABP II”) and Alta Embarcadero BioPharma Partners II, LLC (“AEBP II”). Farah Champsi (known as the “Principal”) is the managing director of Alta BioPharma Management II, LLC (“ABM II”) (which is the general partner of ABP II), and manager of AEBP II. As managing director and manager of such entities, Ms. Champsi may be deemed to have voting and investment power for the shares held by ABP II and AEBP II. The Principal of Alta Partners II, Inc. disclaims beneficial ownership of all such shares held by ABP II and AEBP II, except to the extent of their proportionate pecuniary interests therein. ABM II disclaims beneficial ownership of all such shares held by ABP II and AEBP II, except to the extent of its pecuniary interest therein. The address for Alta Partners II, Inc. is One Embarcadero Center, 37th Floor, San Francisco, California 94111. |
| (2) | Consists of shares issuable upon conversion of 492,611 shares of Series C preferred stock, 885,481 shares of Series D-1 preferred stock, 94,712 shares of Series D-2 preferred stock, and 171,428 shares of common stock issuable pursuant to warrants exercisable within 60 days of December 11, 2013 held by Burrill Biotechnology Capital Fund, L.P. (“Burrill Biotechnology”). Voting and investment decisions for Burrill Biotechnology are made by the unanimous vote of G. Steven Burrill, Bryant Fong, Ann F. Hanham, Victor A. Hebert and Roger Wyse. The address for Burrill Biotechnology is One Embarcadero Center, Suite 2700, San Francisco, California 94111. |
| (3) | Consists of shares issuable upon conversion of 188,679 shares of Series B preferred stock, 342,726 shares of Series C preferred stock, 955,215 shares of Series D-1 preferred stock, 341,456 shares of Series D-2 preferred stock, and 285,712 shares of common stock issuable pursuant to warrants exercisable within 60 days of December 11, 2013 held by F.C.P.R. Genavent. Voting and investment decisions for F.C.P.R. |
136
| Genavent are made by the unanimous vote of Stanislas Cuny, Frederic Exshaw and Amar Douhane. The address for F.C.P.R. Genavent is 90 boulevard Pasteur, CS 21564, Paris Cedex 15, France 75730. |
| (4) | Consists of shares issuable upon conversion of 166,482 shares of Series B preferred stock, 313,996 shares of Series C preferred stock, 863,672 shares of Series D-1 preferred stock, 623,880 shares of Series D-2 preferred stock and 744,676 shares of common stock issuable pursuant to warrants exercisable within 60 days of December 11, 2013 held by FCPR Biotechnology Fund (“FCPR Biotechnology”). Voting and investment decisions for FCPR Biotechnology are made by the unanimous vote of Jean-Yves Nothias and Pierre Gillet. The address for FCPR Biotechnology is 57 Rue de Richelieu, 75002, Paris, France. |
| (5) | Consists of shares issuable upon conversion of 1,739,130 shares of Series C-2 preferred stock held by Merial Limited. Voting and investment decisions for Merial Limited are made by Corsten Hellmann as legal represenative. The address for Merial Limited is 3239 Satellite Boulevard, Duluth, Georgia 30096-4640. |
| (6) | Consists of shares issuable upon conversion of 272,267 shares of Series C preferred stock, 608,696 shares of Series C-2 preferred stock, 762,944 shares of Series D-1 preferred stock, 185,570 shares of Series D-2 preferred stock, and 502,053 shares of common stock issuable pursuant to warrants exercisable within 60 days of December 11, 2013 held by S.R. One, Limited. All of the shares and warrants are held of record by S.R. One, Limited. S.R. One, Limited is a wholly-owned subsidiary of GlaxoSmithKline plc. Generally, voting and investment decisions for S.R. One, Limited are made by a majority ratification, but may deviate from that process in the ordinary course. The address for S.R. One, Limited is 161 Washington Street, Suite 500 Conshohocken, Pennsylvania 19428-2077. |
| (7) | Consists of shares issuable upon conversion of 109,879 shares of Series B preferred stock, 340,509 shares of Series C preferred stock, 809,584 shares of Series D-1 preferred stock, 3,010,807 shares of Series D-2 preferred stock, and 1,463,010 shares of common stock issuable pursuant to warrants exercisable within 60 days of December 11, 2013 held by Ventech Capital II (“Ventech”). Voting and investment decisions for Ventech are made by Alain Caffi. The address for Ventech is 5/7 rue de Monttessuy, Paris Cedex 07, France 75730. |
| (8) | Includes shares issuable upon exercise of options to acquire 609,000 shares of common stock exercisable within 60 days of December 11, 2013. |
| (9) | Consists of shares issuable upon exercise of options to acquire 45,800 shares of common stock exercisable within 60 days of December 11, 2013. |
| (10) | Includes shares issuable upon exercise of options to acquire 153,674 shares of common stock exercisable within 60 days of December 11, 2013. |
| (11) | Consists of shares issuable upon exercise of options to acquire 273,000 shares of common stock exercisable within 60 days of December 11, 2013. |
| (12) | Includes shares issuable upon exercise of options to acquire 75,000 shares of common stock exercisable within 60 days of December 11, 2013. Also includes 76,272 shares of common stock, shares of common stock issuable upon conversion of 8,127 shares of Series D-1 preferred stock, and 1,665 shares of D-2 preferred stock and 5,969 shares of common stock issuable pursuant to warrants exercisable within 60 days of December 11, 2013 held by Valerie Claude Tortelier, Mr. Arthaud’s spouse. |
| (13) | Includes shares held by Ventech. See Note 7. Dr. Chaoui disclaims beneficial ownership of the shares held by Ventech, except to the extent of her ability to direct the voting or disposition of such shares or her pecuniary interest therein. |
137
| (14) | See Note 2. Dr. Hanham disclaims beneficial ownership of the shares held by Burrill Biotechnology, except to the extent of her ability to direct the voting or disposition of such shares or her pecuniary interest therein. |
| (15) | Consists of shares issuable upon exercise of options to acquire 175,000 shares of common stock exercisable within 60 days of December 11, 2013. |
| (16) | See Note 4. Mr. Nothias disclaims beneficial ownership of the shares held by FCPR Biotechnology, except to the extent of his ability to direct the voting or disposition of such shares or his pecuniary interest therein. |
| (17) | Consists of shares held by each executive officer and director including the shares described in footnotes 8 through 16 above. |
138
The following description of our capital stock and certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws are summaries and are qualified by reference to the amended and restated certificate of incorporation and the amended and restated bylaws that will be in effect upon the closing of this offering. Copies of these documents will be filed with the SEC as exhibits to our registration statement, of which this prospectus forms a part. The descriptions of our common stock and convertible preferred stock reflect changes to our capital structure that will occur upon the closing of this offering.
General
Upon the closing of this offering, our amended and restated certificate of incorporation will provide for common stock and will authorize shares of undesignated preferred stock, the rights, preferences and privileges of which may be designated from time to time by our board of directors.
Upon the closing of this offering, our authorized capital stock will consist of shares, all with a par value of $0.001 per share, of which:
| Ÿ | shares are designated as common stock; and |
| Ÿ | shares are designated as preferred stock. |
Common stock
As of December 11, 2013, we had outstanding 40,720,182 shares of common stock, which assumes the conversion of all outstanding shares of convertible preferred stock into shares of common stock immediately prior to the closing of this offering. As of December 11, 2013, we had outstanding 17,414,632 shares of convertible preferred stock, all of which will be converted into 33,904,001 shares of common stock immediately prior to the closing of this offering and 6,816,181 shares of our common stock. Our outstanding capital stock was held by approximately 223 stockholders of record as of December 11, 2013. As of December 11, 2013, we had outstanding options to acquire 1,586,286 shares of common stock and 1,063,242 shares of common stock held by employees, directors and consultants pursuant to our 1999 Stock Plan and 2009 Stock Plan, respectively, having a weighted-average exercise price of $1.16 per share. As of December 11, 2013, we also had outstanding warrants exercisable for 5,235,889 shares of common stock, having an exercise price of $0.01 per share, warrants exercisable for 12,308 shares of our common stock having an exercise price of $3.25 per share and warrants exercisable for 196,923 shares of convertible preferred stock, having an exercise price of $3.25 per share, which will become exercisable for 283,147 shares of common stock immediately prior to the closing of this offering.
Voting Rights
Each holder of our common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of stockholders, except as otherwise expressly provided in our amended and restated certificate of incorporation or required by applicable law. Cumulative voting for the election of directors is not provided for in our amended and restated certificate of incorporation, which means that the holders of a majority of our shares of common stock can elect all of the directors then standing for election.
Dividends and Distributions. Subject to preferences that may apply to any shares of convertible preferred stock outstanding at the time, the holders of outstanding shares of our common stock are entitled to receive dividends out of funds legally available at the times and in the amounts that our board of directors may determine.
139
Liquidation Rights. Upon our liquidation, dissolution or winding-up, the assets legally available for distribution to our stockholders would be distributable ratably among the holders of our common stock and any participating convertible preferred stock outstanding at that time after payment of liquidation preferences, if any, on any outstanding shares of convertible preferred stock and payment of other claims of creditors.
The rights, preferences, and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of holders of shares of any series of convertible preferred stock that we may designate and issue in the future.
Preemptive or Similar Rights. Our common stock is not entitled to preemptive rights and is not subject to conversion, redemption or sinking fund provisions.
Preferred Stock
As of December 11, 2013, there were 17,414,632 shares of our convertible preferred stock outstanding and warrants exercisable for 196,923 shares of our preferred stock with an exercise price of $3.25 per share. Immediately prior to the closing of this offering, all outstanding shares of our convertible preferred stock will convert into 33,904,001 shares of our common stock.
Upon the closing of this offering, our board of directors may, without further action by our stockholders, fix the rights, preferences, privileges and restrictions of up to an aggregate of shares of preferred stock in one or more series and authorize their issuance. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of our common stock. The issuance of our preferred stock could adversely affect the voting power of holders of our common stock and the likelihood that these holders will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change of control or other corporate action. Upon the closing of this offering, no shares of preferred stock will be outstanding, and we have no present plan to issue any shares of preferred stock.
Warrants
As of December 11, 2013, we had warrants to purchase an aggregate of 5,235,889 shares of our common stock outstanding with an exercise price of $0.01 per share. Each of these warrants has a net exercise provision under which the holder, in lieu of payment of the exercise price in cash, can surrender the warrant and receive a net number of shares of our common stock based on the fair market value of such stock at the time of exercise of the warrant after deducting of the aggregate exercise price. Unless earlier exercised, these warrants will expire upon the closing of this offering.
As of December 11, 2013, we had a warrant to purchase an aggregate of 12,308 shares of our common stock outstanding with an exercise price of $3.25 per share. This warrant has a net exercise provision under which the holder, in lieu of payment of the exercise price in cash, can surrender the warrant and receive a net number of shares of our common stock based on the fair market value of such stock at the time of exercise of the warrant after deduction of the aggregate exercise price. Unless earlier exercised, this warrant will expire on the earlier of September 14, 2014 or five years after the closing of this offering.
As of December 11, 2013, we had warrants to purchase an aggregate of 196,923 shares of our Series C-1 convertible preferred stock outstanding with an exercise price of $3.25 per share. Each of these warrants has a net exercise provision under which the holder, in lieu of payment of the exercise price in cash, can surrender the warrant and receive a net number of shares of Series C-1 convertible preferred stock
140
based on the fair market value of such stock at the time of exercise of the warrant after deduction of the aggregate exercise price. Unless earlier exercised, these warrants will expire on the later of July 14, 2016 or five years after the closing of this offering. Upon the closing of this offering, these warrants will become exercisable for 283,147 shares of our common stock with an exercise price of $2.26 per share.
Registration Rights
Stockholder Registration Rights
We are party to an investor rights agreement which provides that holders of our convertible preferred stock have certain registration rights, as set forth below. This investor rights agreement was entered into in August 2000 and has been amended and/or restated from time to time in connection with our preferred stock financings. The registration of shares of our common stock pursuant to the exercise of registration rights described below would enable the holders to sell these shares without restriction under the Securities Act of 1933, as amended, or the Securities Act, when the applicable registration statement is declared effective. We will pay the registration expenses, other than underwriting discounts and commissions, of the shares registered pursuant to the demand, piggyback and Form S-3 registrations described below.
Generally, in an underwritten offering, the managing underwriter, if any, has the right, subject to specified conditions, to limit the number of shares such holders may include. The demand, piggyback and Form S-3 registration rights described below will expire the later of (1) three years after the effective date of the registration statement containing this prospectus or (2) with respect to each stockholder, at such time as the (A) our capital stock is publicly traded and (B) such stockholder holds less than one percent (1%) of the our common stock outstanding and is entitled to sell all of its shares pursuant to Rule 144 of the Securities Act during any ninety (90) day period.
Demand Registration Rights
The holders of an aggregate of 41,947,651 shares of our common stock issuable upon conversion of outstanding convertible preferred stock will be entitled to certain demand registration rights. At any time beginning 180 days after the closing of this offering, the holders of forty percent (40%) of these shares may request that we file a registration statement having an aggregate offering price to the public of not less than $5.0 million to register all or a portion of their shares.
Piggyback Registration Rights
In connection with this offering, the holders of an aggregate of 43,653,423 shares of our common stock, issuable (1) upon conversion of outstanding convertible preferred stock, (2) upon exercise of outstanding common stock warrants, and (3) conversion of preferred stock currently subject to outstanding warrants, were entitled to, and the necessary percentage of holders waived, their rights to include their shares of registrable securities in this offering. In the event that we propose to register any of our securities under the Securities Act either for our own account or for the account of other security holders, the holders of these shares will be entitled to certain “piggyback” registration rights allowing them to include their shares in the registration, subject to certain marketing and other limitations. As a result, whenever we propose to file a registration statement under the Securities Act including a registration statement on Form S-3 as discussed below, other than with respect to a demand registration or a registration statement on Forms S-4 or S-8, the holders of these shares are entitled to notice of the registration and have the right, subject to limitations that the underwriters may impose on the number of shares included in the registration, to include their shares in the registration.
141
Form S-3 Registration Rights
The holders of an aggregate of 43,653,423 shares of our common stock, issuable upon (1) conversion of outstanding convertible preferred stock, (2) exercise of outstanding common stock warrants, and (3) conversion of preferred stock currently subject to outstanding warrants will be entitled to certain Form S-3 registration rights. These holders may make a request that we register their shares on Form S-3 if we are qualified to file a registration statement on Form S-3. The request for registration on Form S-3 must cover securities the aggregate offering price of which, before payment of underwriting discounts and commissions, is at least $1,000,000.
Anti-Takeover Provisions
Certificate of Incorporation and Bylaws to be in Effect Upon the Closing of This Offering
Because our stockholders do not have cumulative voting rights, our stockholders holding a majority of the voting power of our shares of common stock outstanding will be able to elect all of our directors. Our amended and restated certificate of incorporation and amended and restated bylaws to be effective upon the closing of this offering will provide that all stockholder actions must be effected at a duly called meeting of stockholders and not by consent in writing. A special meeting of stockholders may be called only by a majority of our whole board of directors, the chair of our board of directors, or our chief executive officer.
Our amended and restated certificate of incorporation will further provide that, immediately after this offering, the affirmative vote of holders of at least sixty-six and two-thirds percent (66-2/3%) of the voting power of all of the then outstanding shares of voting stock, voting as a single class, will be required to amend certain provisions of our certificate of incorporation, including provisions relating to the size of the board, removal of directors, special meetings, actions by written consent and cumulative voting. The affirmative vote of holders of at least sixty-six and two-thirds percent (66-2/3%) of the voting power of all of the then outstanding shares of voting stock, voting as a single class, will be required to amend or repeal our bylaws, although our bylaws may be amended by a simple majority vote of our board of directors.
The foregoing provisions will make it more difficult for our existing stockholders to replace our board of directors as well as for another party to obtain control of our company by replacing our board of directors. Since our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management. In addition, the authorization of undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change the control of our company.
These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage certain types of transactions that may involve an actual or threatened acquisition of our company. These provisions are also designed to reduce our vulnerability to an unsolicited acquisition proposal and to discourage certain tactics that may be used in proxy rights. However, these provisions could have the effect of discouraging others from making tender offers for our shares and may have the effect of deterring hostile takeovers or delaying changes in control of our company or our management. As a consequence, these provisions also may inhibit fluctuations in the market price of our stock that could result from actual or rumored takeover attempts.
142
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
| Ÿ | before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| Ÿ | upon closing of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned by (1) persons who are directors and also officers and (2) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| Ÿ | on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines business combination to include the following:
| Ÿ | any merger or consolidation involving the corporation and the interested stockholder; |
| Ÿ | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| Ÿ | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| Ÿ | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or |
| Ÿ | the receipt by the interested stockholder of the benefit of any loss, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Limitations on Liability and Indemnification
See the section of this prospectus titled “Executive Compensation—Limitation on Liability and Indemnification Matters.”
Listing
We will be applying to have our common stock to be approved for listing on the NASDAQ Global Market under the trading symbol “SCYX.”
Transfer Agent and Registrar
Upon the closing of this offering, the transfer agent and registrar for our common stock will be .
143
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our capital stock. Future sales of our common stock in the public market, or the availability of such shares for sale in the public market, could adversely affect market prices prevailing from time to time. As described below, only a limited number of shares will be available for sale shortly after this offering due to contractual and legal restrictions on resale. Nevertheless, sales of our common stock in the public market after such restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing market price at such time and our ability to raise equity capital in the future.
Based on the number of shares outstanding as of December 31, 2013, upon the closing of this offering, shares of our common stock will be outstanding, assuming no exercise of the underwriters’ over-allotment option to purchase additional shares of common stock and no exercise of outstanding options or warrants. Of the outstanding shares, all of the shares sold in this offering will be freely tradable, except that any shares held by our affiliates, as that term is defined in Rule 144 under the Securities Act of 1933, as amended, or the Securities Act, may only be sold in compliance with the limitations described below.
The remaining shares of our common stock outstanding after this offering are restricted securities as such term is defined in Rule 144 under the Securities Act or are subject to lock-up agreements with us as described below. Following the expiration of the lock-up period, restricted securities may be sold in the public market only if registered or if they qualify for an exemption from registration under Rule 144 or 701 promulgated under the Securities Act described in greater detail below.
Rule 144
In general, a person who has beneficially owned restricted shares of our common stock for at least six months would be entitled to sell their securities provided that (a) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding a sale and (b) we are subject to the periodic reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, for at least 90 days before the sale. Persons who have beneficially owned restricted shares of our common stock for at least six months but who are our affiliates at the time of, or any time during the 90 days preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of either of the following:
| Ÿ | 1% of the number of shares of our common stock outstanding after this offering, which will equal approximately shares immediately after the closing of this offering, based on the number of common shares outstanding as of December 31, 2013, and assuming no exercise of the underwriters’ over-allotment option to purchase additional shares of our common stock; or |
| Ÿ | the average weekly trading volume of our common stock on the during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale; |
provided, in each case, that we are subject to the periodic reporting requirements of the Exchange Act, for at least 90 days before the sale. Such sales both by affiliates and by non-affiliates must also comply with the manner of sale, current public information and notice provisions of Rule 144.
Rule 701
Rule 701 under the Securities Act as in effect on the date of this prospectus, permits resales of shares in reliance upon Rule 144 but without compliance with certain restrictions of Rule 144, including the holding period requirement. Most of our employees, executive officers, directors or consultants who
144
purchased shares under a written compensatory plan or contract may be entitled to rely on the resale provisions of Rule 701, but all holders of Rule 701 shares are required to wait until 90 days after the date of this prospectus before selling their shares. However, substantially all Rule 701 shares are subject to lock-up agreements as described below and under the section of this prospectus titled “Underwriting” and will not become eligible for sale until the expiration of those agreements.
Lock-up Agreements
We, our directors and officers, and substantially all of our stockholders and optionholders have agreed with the underwriters that for a period of 180 days following the date of this prospectus, not to offer, sell, assign, transfer, pledge, contract to sell or otherwise dispose of or hedge any shares of our common stock or any securities convertible into or exchangeable for shares of our common stock, subject to specified exceptions. RBC Capital Markets, LLC may, in its sole discretion, at any time, release all or any portion of the shares from the restrictions in these agreements.
Registration Rights
On the date beginning 180 days after the date of this prospectus, the holders of approximately 43,653,423 shares of our common stock, or their transferees, will be entitled to certain rights with respect to the registration of those shares under the Securities Act. For a description of these registration rights, see the section of this prospectus titled “Description of Capital Stock—Registration Rights.” If these shares are registered, they will be freely tradable without restriction under the Securities Act.
Equity Incentive Plans
As soon as practicable after the closing of this offering, we intend to file a Form S-8 registration statement under the Securities Act, to register shares of our common stock issued or reserved for issuance under our equity compensation plans and agreements. This registration statement will become effective immediately upon filing, and shares covered by this registration statement will thereupon be eligible for sale in the public markets, subject to vesting restrictions, the lock-up agreements described above and Rule 144 limitations applicable to affiliates. For a more complete discussion of our equity compensation plans, see the section of this prospectus titled “Executive Compensation—Equity Incentive Plans.”
145
MATERIAL U.S. FEDERAL INCOME AND ESTATE TAX CONSIDERATIONS FOR NON-U.S. HOLDERS
The following summary describes the material U.S. federal income and estate tax consequences of the acquisition, ownership and disposition of our common stock acquired in this offering by Non-U.S. Holders (as defined below). This discussion does not address all aspects of U.S. federal income and estate taxes and does not deal with foreign, state and local consequences that may be relevant to Non-U.S. Holders in light of their particular circumstances, nor does it address U.S. federal tax consequences other than income and estate taxes. Special rules different from those described below may apply to certain Non-U.S. Holders that are subject to special treatment under the Internal Revenue Code of 1986, as amended, or the Code, such as financial institutions, insurance companies, tax-exempt organizations, broker-dealers and traders in securities, U.S. expatriates, “controlled foreign corporations,” “passive foreign investment companies,” corporations that accumulate earnings to avoid U.S. federal income tax, persons that hold our common stock as part of a “straddle,” “hedge,” “conversion transaction,” “synthetic security” or integrated investment or other risk reduction strategy, persons subject to the alternative minimum tax or Medicare contribution tax, partnerships and other pass-through entities, and investors in such pass-through entities. Such Non-U.S. Holders are urged to consult their own tax advisors to determine the U.S. federal, state, local and other tax consequences that may be relevant to them. Furthermore, the discussion below is based upon the provisions of the Code, and Treasury regulations, rulings and judicial decisions thereunder as of the date hereof, and such authorities may be repealed, revoked or modified, perhaps retroactively, so as to result in U.S. federal income and estate tax consequences different from those discussed below. We have not requested a ruling from the U.S. Internal Revenue Service, or IRS, with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS will agree with such statements and conclusions. This discussion assumes that the Non-U.S. Holder holds our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment).
Persons considering the purchase of our common stock pursuant to this offering should consult their own tax advisors concerning the U.S. federal income and estate tax consequences of acquiring, owning and disposing of our common stock in light of their particular situations as well as any consequences arising under the laws of any other taxing jurisdiction, including any state, local or foreign tax consequences.
For the purposes of this discussion, a “Non-U.S. Holder” is, for U.S. federal income tax purposes, a beneficial owner of common stock that is neither a U.S. Holder, nor a partnership (or other entity treated as a partnership for U.S. federal income tax purposes regardless of its place of organization or formation). A “U.S. Holder” means a beneficial owner of our common stock that is for U.S. federal income tax purposes (a) an individual who is a citizen or resident of the U.S., (b) a corporation or other entity treated as a corporation created or organized in or under the laws of the U.S., any state thereof or the District of Columbia, (c) an estate the income of which is subject to U.S. federal income taxation regardless of its source or (d) a trust if it (1) is subject to the primary supervision of a court within the U.S. and one or more U.S. persons have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
Distributions
Subject to the discussion below, distributions, if any, made on our common stock to a Non-U.S. Holder of our common stock to the extent made out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles) generally will constitute dividends for U.S. tax purposes and will be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. To obtain a reduced rate of withholding under a treaty, a Non-U.S. Holder
146
generally will be required to provide us with a properly executed IRS Form W-8BEN, or other appropriate form, certifying the Non-U.S. Holder’s entitlement to benefits under that treaty. In the case of a Non-U.S. Holder that is an entity, Treasury Regulations and the relevant tax treaty provide rules to determine whether, for purposes of determining the applicability of a tax treaty, dividends will be treated as paid to the entity or to those holding an interest in that entity. If a Non-U.S. Holder holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent will then be required to provide certification to us or our paying agent, either directly or through other intermediaries. If you are eligible for a reduced rate of U.S. federal withholding tax under an income tax treaty, you may be able to obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS.
We generally are not required to withhold tax on dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the U.S. (and, if required by an applicable income tax treaty, are attributable to a permanent establishment that such holder maintains in the U.S.) if a properly executed IRS Form W-8ECI, stating that the dividends are so connected, is furnished to us (or, if stock is held through a financial institution or other agent, to such agent). In general, such effectively connected dividends will be subject to U.S. federal income tax, on a net income basis at the regular graduated rates. A corporate Non-U.S. Holder receiving effectively connected dividends may also be subject to an additional “branch profits tax,” which is imposed, under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) on the corporate Non-U.S. Holder’s effectively connected earnings and profits, subject to certain adjustments.
To the extent distributions on our common stock, if any, exceed our current and accumulated earnings and profits, they will first reduce the Non-U.S. Holder’s adjusted basis in our common stock, but not below zero, and then will be treated as gain to the extent of any excess, and taxed in the same manner as gain realized from a sale or other disposition of common stock as described in the next section.
Gain on Disposition of Our Common Stock
Subject to the discussion below regarding backup withholding and foreign accounts, a Non-U.S. Holder generally will not be subject to U.S. federal income tax with respect to gain realized on a sale or other disposition of our common stock unless (a) the gain is effectively connected with a trade or business of such holder in the U.S. (and, if required by an applicable income tax treaty, is attributable to a permanent establishment that such holder maintains in the U.S.), (b) the Non-U.S. Holder is a nonresident alien individual and is present in the U.S. for 183 or more days in the taxable year of the disposition and certain other conditions are met or (c) we are or have been a “United States real property holding corporation” within the meaning of Code Section 897(c)(2) at any time within the shorter of the five-year period preceding such disposition or such holder’s holding period. In general, we would be a U.S. real property holding corporation if interests in U.S. real estate comprised (by fair market value) at least half of our business assets. We believe that we are not, and do not anticipate becoming, a U.S. real property holding corporation. Even if we are treated as a U.S. real property holding corporation, gain realized by a Non-U.S. Holder on a disposition of our common stock will not be subject to U.S. federal income tax so long as (1) the Non-U.S. Holder owned, directly, indirectly and constructively, no more than five percent of our common stock at all times within the shorter of (i) the five-year period preceding the disposition or (ii) the holder’s holding period and (2) our common stock is regularly traded on an established securities market. There can be no assurance that our common stock will continue to qualify as regularly traded on an established securities market.
If you are a Non-U.S. Holder described in (a) above, you will be required to pay tax on the net gain derived from the sale at regular graduated U.S. federal income tax rates, and corporate Non-U.S. Holders described in (a) above may be subject to the additional branch profits tax at a 30% rate or such lower rate as
147
may be specified by an applicable income tax treaty. If you are an individual Non-U.S. Holder described in (b) above, you will be required to pay a flat 30% tax on the gain derived from the sale, which gain may be offset by U.S. source capital losses (even though you are not considered a resident of the U.S.).
Information Reporting Requirements and Backup Withholding
Generally, we must report information to the IRS with respect to any dividends we pay on our common stock including the amount of any such dividends, the name and address of the recipient, and the amount, if any, of tax withheld. A similar report is sent to the holder to whom any such dividends are paid. Pursuant to tax treaties or certain other agreements, the IRS may make its reports available to tax authorities in the recipient’s country of residence.
Dividends paid by us (or our paying agents) to a Non-U.S. Holder may also be subject to U.S. backup withholding. U.S. backup withholding generally will not apply to a Non-U.S. Holder who provides a properly executed IRS Form W-8BEN or otherwise establishes an exemption.
Under current U.S. federal income tax law, U.S. information reporting and backup withholding requirements generally will apply to the proceeds of a disposition of our common stock effected by or through a U.S. office of any broker, U.S. or foreign, except that information reporting and such requirements may be avoided if the holder provides a properly executed IRS Form W-8BEN or otherwise meets documentary evidence requirements for establishing Non-U.S. Holder status or otherwise establishes an exemption. Generally, U.S. information reporting and backup withholding requirements will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the U.S. through a non-U.S. office of a non-U.S. broker. Information reporting and backup withholding requirements may, however, apply to a payment of disposition proceeds if the broker has actual knowledge, or reason to know, that the holder is, in fact, a U.S. person. For information reporting purposes, certain brokers with substantial U.S. ownership or operations will generally be treated in a manner similar to U.S. brokers.
Any amounts of tax withheld under the backup withholding rules may be credited against the tax liability of persons subject to backup withholding, provided that the required information is timely furnished to the IRS.
Foreign Accounts
A U.S. federal withholding tax of 30% may apply on dividends on and the gross proceeds of a disposition of our common stock paid to a foreign financial institution (as specifically defined by applicable rules) unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which includes certain equity holders of such institution, as well as certain account holders that are foreign entities with U.S. owners). This U.S. federal withholding tax of 30% will also apply on dividends on and the gross proceeds of a disposition of our common stock to a non-financial foreign entity unless such entity provides the withholding agent with either a certification that it does not have any substantial direct or indirect U.S. owners or provides information regarding substantial direct and indirect U.S. owners of the entity. The withholding tax described above will not apply if the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from the rules. Under certain circumstances, a Non-U.S. Holder might be eligible for refunds or credits of such taxes. Holders are encouraged to consult with their own tax advisors regarding the possible implications of these rules for their investment in our common stock.
The IRS has issued guidance providing that the withholding provisions described above will generally apply to payments of dividends made on or after July 1, 2014 and to payments of gross proceeds from a sale or other disposition of common stock on or after January 1, 2017.
148
Federal Estate Tax
An individual Non-U.S. Holder who is treated as the owner of, or has made certain lifetime transfers of, an interest in our common stock will be required to include the value thereof in his or her gross estate for U.S. federal estate tax purposes, and may be subject to U.S. federal estate tax unless an applicable estate tax treaty provides otherwise, even though such individual was not a citizen or resident of the U.S. at the time of his or her death.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAW.
149
Subject to the terms and conditions set forth in the underwriting agreement, dated , 2014, between us and RBC Capital Markets, LLC, as the representative of the underwriters named below and the book-running manager of this offering, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the respective number of shares of common stock shown opposite its name below:
| UNDERWRITER |
NUMBER OF SHARES | |
| RBC Capital Markets, LLC |
||
|
| ||
| Total |
||
|
|
The underwriting agreement provides that the obligations of the several underwriters are subject to certain conditions precedent such as the receipt by the underwriters of officers’ certificates and legal opinions and approval of certain legal matters by their counsel. The underwriting agreement provides that the underwriters will purchase all of the shares of common stock if any of them are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the nondefaulting underwriters may be increased or the underwriting agreement may be terminated. We have agreed to indemnify the underwriters and certain of their controlling persons against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make in respect of those liabilities.
The underwriters have advised us that, following the completion of this offering, they currently intend to make a market in the common stock as permitted by applicable laws and regulations. However, the underwriters are not obligated to do so, and the underwriters may discontinue any market-making activities at any time without notice in their sole discretion. Accordingly, no assurance can be given as to the liquidity of the trading market for the common stock, that you will be able to sell any of the common stock held by you at a particular time or that the prices that you receive when you sell will be favorable.
The underwriters are offering the shares of common stock subject to their acceptance of the shares of common stock from us and subject to prior sale. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part. In addition, the underwriters have advised us that they do not expect sales to accounts over which they have discretionary authority to exceed 5% of the common stock being offered.
Commissions and Expenses
The underwriters have advised us that they propose to offer the shares of common stock to the public at the initial public offering price set forth on the cover page of this prospectus and to certain dealers, which may include the underwriters, at that price less a concession not in excess of $ per share of common stock. After the offering, the initial public offering price, the concession to dealers or any other term of the offering may be changed by the representative. No such change will change the amount of proceeds to be received by us as set forth on the cover page of this prospectus.
150
The following table shows the public offering price, the underwriting discounts and commissions that we are to pay the underwriters and the proceeds, before expenses, to us in connection with this offering. Such amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Per Share | Total | |||||||||||||||
| Without Option to Purchase Additional Shares |
With Option to Purchase Additional Shares |
Without Option to Purchase Additional Shares |
Without Option to Purchase Additional Shares |
|||||||||||||
| Public offering price |
$ | $ | $ | $ | ||||||||||||
| Underwriting discounts and commissions paid by us |
$ | $ | $ | $ | ||||||||||||
| Proceeds to us, before expenses |
$ | $ | $ | $ | ||||||||||||
We estimate expenses payable by us in connection with this offering, other than the underwriting discounts and commissions referred to above, will be approximately $ million.
Determination of Offering Price
Prior to this offering, there has not been a public market for our common stock. Consequently, the initial public offering price for our common stock will be determined by negotiations between us and the representative. Among the factors to be considered in these negotiations will be prevailing market conditions, our financial information, market valuations of other companies that we and the underwriters believe to be comparable to us, estimates of our business potential, the present state of our development and other factors deemed relevant.
We offer no assurances that the initial public offering price will correspond to the price at which the common stock will trade in the public market subsequent to the offering or that an active trading market for the common stock will develop and continue after the offering.
Listing
We have applied to list our common stock on the NASDAQ Global Market under the symbol “SCYX.”
Option to Purchase Additional Shares
We have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase, from time to time, in whole or in part, up to an aggregate of shares from us at the public offering price set forth on the cover page of this prospectus, less underwriting discounts and commissions, to cover over-allotments. If the underwriters exercise this option, each underwriter will be obligated, subject to specified conditions, to purchase a number of additional shares proportionate to that underwriter’s initial purchase commitment as indicated in the table above.
No Sales of Similar Securities
Pursuant to certain lock-up agreements, we, our officers, directors and holders of substantially all of our outstanding capital stock have agreed, subject to specified exceptions, not to directly or indirectly:
| Ÿ | sell, offer to sell, contract to sell, effect any short sale, pledge, transfer, establish a “put equivalent position” within the meaning of Rule 16a-l(h) under the Exchange Act, or |
151
| Ÿ | otherwise dispose of, or enter into any swap, hedge or similar arrangement that transfers, in whole or in part, the economic risk of ownership of, any shares of common stock, options or warrants to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock currently or hereafter owned either of record or beneficially, or |
| Ÿ | make any demand for, or exercise any right with respect to, the registration under the Securities Act of the offer and sale of any shares of common stock, options or warrants to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock, or |
| Ÿ | publicly announce any intention to do any of the foregoing |
for a period of 180 days after the date of this prospectus without the prior written consent of the representative, subject to specified exceptions.
This restriction terminates after the close of trading of the common stock on and including the 180th day after the date of this prospectus. The representative may, in its sole discretion and at any time or from time to time before the termination of the 180-day period release all or any portion of the securities subject to lock-up agreements. There are no existing agreements between the underwriters and any of our stockholders who will execute a lock-up agreement providing consent to the sale of shares prior to the expiration of the lock-up period.
Stabilization
The underwriters have advised us that, pursuant to Regulation M under the Exchange Act, certain persons participating in the offering may engage in short sale transactions, stabilizing transactions, syndicate covering transactions or the imposition of penalty bids in connection with this offering. These activities may have the effect of stabilizing or maintaining the market price of the common stock at a level above that which might otherwise prevail in the open market. Establishing short sales positions may involve either “covered” short sales or “naked” short sales.
“Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares of our common stock in this offering. The underwriters may close out any covered short position by either exercising their option to purchase additional shares of our common stock or purchasing shares of our common stock in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option to purchase additional shares.
“Naked” short sales are sales in excess of the option to purchase additional shares of our common stock. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares of our common stock in the open market after pricing that could adversely affect investors who purchase in this offering.
A stabilizing bid is a bid for the purchase of shares of common stock on behalf of the underwriters for the purpose of fixing or maintaining the price of the common stock. A syndicate covering transaction is the bid for or the purchase of shares of common stock on behalf of the underwriters to reduce a short position incurred by the underwriters in connection with the offering. Similar to other purchase transactions, the underwriter’s purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. A penalty bid is an arrangement permitting the underwriters to reclaim the selling
152
concession otherwise accruing to a syndicate member in connection with the offering if the common stock originally sold by such syndicate member are purchased in a syndicate covering transaction and therefore have not been effectively placed by such syndicate member.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. The underwriters are not obligated to engage in these activities and, if commenced, any of the activities may be discontinued at any time.
Electronic Distribution
A prospectus in electronic format may be made available by e-mail or through online services maintained by one or more of the underwriters or their affiliates. In those cases, prospective investors may view offering terms online and may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of shares of common stock for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriters on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters’ web sites and any information contained in any other web site maintained by any of the underwriters is not part of this prospectus, has not been approved and/or endorsed by us or the underwriters and should not be relied upon by investors.
Other Activities and Relationships
The underwriters and certain of their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters and certain of their respective affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the underwriters and certain of their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments issued by us and our affiliates. If the underwriters or their respective affiliates have a lending relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The underwriters and their respective affiliates may hedge such exposure by entering into transactions which consist of either the purchase of credit default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially the common stock offered hereby. Any such short positions could adversely affect future trading prices of the common stock offered hereby. The underwriters and certain of their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Notice to Residents of Canada
The securities may be sold only to purchasers purchasing as principal that are both “accredited investors” as defined in National Instrument 45-106 Prospectus and Registration Exemptions and “permitted clients” as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from the prospectus requirements and in compliance with the registration requirements of applicable securities laws.
153
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended, which we refer to as the Order, and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). This document must not be acted on or relied on in the United Kingdom by persons who are not relevant persons. In the United Kingdom, any investment or investment activity to which this document relates is only available to, and will be engaged in with, relevant persons.
Notice to Prospective Investors in the EEA
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive, which we refer to as a Relevant Member State, an offer to the public of any shares of common stock that are the subject of the offering contemplated by this prospectus may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any shares may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
| (a) | to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| (b) | to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; |
| (c) | by the underwriters to fewer than 100 natural or legal persons (other than “qualified investors” as defined in the Prospectus Directive) subject to obtaining the prior consent of the representative for any such offer; or |
| (d) | in any other circumstances falling within Article 3(2) of the Prospectus Directive; |
provided that no such offer of shares shall result in a requirement for the publication by us or any representative of a prospectus pursuant to Article 3 of the Prospectus Directive.
Any person making or intending to make any offer of shares within the EEA should only do so in circumstances in which no obligation arises for us or any of the underwriters to produce a prospectus for such offer. Neither we nor the underwriters have authorized, nor do they authorize, the making of any offer of shares through any financial intermediary, other than offers made by the underwriters which constitute the final offering of shares contemplated in this prospectus.
For the purposes of this provision, and your representation below, the expression an “offer to the public” in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase any shares, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
154
Each person in a Relevant Member State who receives any communication in respect of, or who acquires any shares under, the offer of shares contemplated by this prospectus will be deemed to have represented, warranted and agreed to and with us and each underwriter that:
| (a) | it is a “qualified investor” within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive; and |
| (b) | in the case of any shares acquired by it as a financial intermediary, as that term is used in Article 3(2) of the Prospectus Directive, (i) the shares acquired by it in the offering have not been acquired on behalf of, nor have they been acquired with a view to their offer or resale to, persons in any Relevant Member State other than “qualified investors” (as defined in the Prospectus Directive), or in circumstances in which the prior consent of the representative has been given to the offer or resale; or (ii) where shares have been acquired by it on behalf of persons in any Relevant Member State other than qualified investors, the offer of those shares to it is not treated under the Prospectus Directive as having been made to such persons. |
Notice to Prospective Investors in Switzerland
The Prospectus does not constitute an issue prospectus pursuant to Article 652a or Article 1156 of the Swiss Code of Obligations (“CO”) and the shares will not be listed on the SIX Swiss Exchange. Therefore, the Prospectus may not comply with the disclosure standards of the CO and/or the listing rules (including any prospectus schemes) of the SIX Swiss Exchange. Accordingly, the shares may not be offered to the public in or from Switzerland, but only to a selected and limited circle of investors, which do not subscribe to the shares with a view to distribution.
155
Cooley LLP, Palo Alto, California, will pass upon the validity of the shares of common stock offered hereby. The underwriters are being represented by DLA Piper LLP (US), East Palo Alto, California, in connection with this offering.
The financial statements as of December 31, 2012 and 2011, and for each of the two years in the period ended December 31, 2012, included in this prospectus have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report appearing herein and elsewhere in the Registration Statement (which report expresses an unqualified opinion on the financial statements and includes explanatory paragraphs referring to going concern uncertainty and a restatement of the 2012 and 2011 financial statements). Such financial statements have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to this offering of our common stock. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement, some items of which are contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and our common stock, we refer you to the registration statement, including the exhibits and the financial statements and notes filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract or any other document are not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please see the copy of the contract or document that has been filed. Each statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit. The exhibits to the registration statement should be referenced for the complete contents of these contracts and documents. A copy of the registration statement and the exhibits filed therewith may be inspected without charge at the public reference room of the SEC, located at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the public reference rooms by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy statements, and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
As a result of this offering, we will become subject to the information and reporting requirements of the Exchange Act and, in accordance with this law, will file periodic and current reports, proxy statements, and other information with the SEC. These periodic and current reports, proxy statements, and other information will be available for inspection and copying at the SEC’s public reference facilities and the website of the SEC referred to above. We also maintain a website at www.scynexis.com. After the closing of this offering, you may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not incorporated by reference into this prospectus.
156
INDEX TO FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
SCYNEXIS, Inc.
Durham, North Carolina
We have audited the accompanying balance sheets of SCYNEXIS, Inc. (the “Company”) as of December 31, 2012 and 2011, and the related statements of operations, changes in convertible preferred stock and stockholders’ deficit, and cash flows for each of the two years in the period ended December 31, 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such financial statements present fairly, in all material respects, the financial position of SCYNEXIS, Inc., as of December 31, 2012 and 2011, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2012, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has experienced recurring losses from operations and negative cash flows. The Company also has negative working capital and debt that will become due in December 2014. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans concerning these matters are also discussed in Note 1 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
As discussed in Note 18 to the financial statements, the accompanying 2012 and 2011 financial statements have been restated to correct misstatements.
/s/ Deloitte & Touche
Raleigh, North Carolina
December 20, 2013
F-2
SCYNEXIS, INC.
(in thousands, except share and per share data)
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| (As Restated) | (As Restated) | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 2,385 | $ | 3,976 | ||||
| Accounts receivable, net of allowance for bad debts |
1,661 | 1,616 | ||||||
| Unbilled services |
757 | 168 | ||||||
| Prepaid expenses and other current assets |
421 | 480 | ||||||
|
|
|
|
|
|||||
| Total current assets |
5,224 | 6,240 | ||||||
| Property and equipment, net of accumulated depreciation |
6,284 | 7,412 | ||||||
| Deferred financing costs |
530 | 2,835 | ||||||
| Other assets |
80 | 98 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 12,118 | $ | 16,585 | ||||
|
|
|
|
|
|||||
| Liabilities and stockholders’ deficit |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 1,018 | $ | 675 | ||||
| Accrued expenses |
811 | 1,562 | ||||||
| Deferred revenue |
182 | 225 | ||||||
| Interest payable — related party |
776 | 29 | ||||||
| Convertible notes — related party, net of discount |
11,444 | 5,215 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
14,231 | 7,706 | ||||||
| Long-term debt |
15,000 | 15,000 | ||||||
| Derivative liability |
683 | 540 | ||||||
| Deferred rent |
1,533 | 1,559 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
31,447 | 24,805 | ||||||
| Commitments and contingencies (Note 8) |
||||||||
| Series A convertible preferred stock, $0.001 par value, authorized 31,410 shares; 31,407 shares issued and outstanding as of December 31, 2012 and 2011 |
250 | 250 | ||||||
| Series B convertible preferred stock, $0.001 par value, authorized 711,987 shares; 467,814 and 711,987 shares issued and outstanding as of December 31, 2012 and 2011, respectively |
4,215 | 6,415 | ||||||
| Series C convertible preferred stock, $0.001 par value, authorized 2,967,678 shares; 2,770,633 and 2,967,678 shares issued and outstanding as of December 31, 2012 and 2011, respectively |
28,121 | 30,121 | ||||||
| Series C-1 convertible preferred stock, $0.001 par value, authorized 3,076,923 shares; 0 and 984,615 shares issued and outstanding as of December 31, 2012 and 2011, respectively |
— | 3,200 | ||||||
| Series C-2 convertible preferred stock, $0.001 par value, authorized 2,347,826 shares; 2,347,826 shares issued and outstanding as of December 31, 2012 and 2011 |
13,500 | 13,500 | ||||||
| Series D-1 convertible preferred stock, $0.001 par value, authorized 5,000,000 shares; 0 shares issued and outstanding as of December 31, 2012 and 2011 |
— | — | ||||||
| Series D-2 convertible preferred stock, $0.001 par value, authorized 5,000,000 shares; 0 shares issued and outstanding as of December 31, 2012 and 2011 |
— | — | ||||||
| Stockholders’ deficit: |
||||||||
| Common stock, $0.001 par value, authorized 54,000,000 shares; issued and outstanding, 6,851,149 and 4,067,347 shares as of December 31, 2012 and 2011, respectively |
7 | 4 | ||||||
| Additional paid-in capital |
17,394 | 9,629 | ||||||
| Accumulated deficit |
(82,816 | ) | (71,339 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ deficit |
(65,415 | ) | (61,706 | ) | ||||
|
|
|
|
|
|||||
| Total liabilities, convertible preferred stock and stockholders’ deficit |
$ | 12,118 | $ | 16,585 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of the financial statements.
F-3
SCYNEXIS, INC.
(in thousands, except share and per share data)
| Year ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| (As Restated) | (As Restated) | |||||||
| Revenue — related party |
$ | 7,424 | $ | 13,618 | ||||
| Revenue |
9,413 | 12,836 | ||||||
|
|
|
|
|
|||||
| Total revenue |
16,837 | 26,454 | ||||||
| Cost of revenue |
14,364 | 17,753 | ||||||
|
|
|
|
|
|||||
| Gross profit |
2,473 | 8,701 | ||||||
|
|
|
|
|
|||||
| Operating expenses: |
||||||||
| Research and development |
8,927 | 11,633 | ||||||
| Selling, general and administrative |
4,742 | 4,980 | ||||||
| Gain on sale of asset |
(3,412 | ) | — | |||||
|
|
|
|
|
|||||
| Total operating expenses |
10,257 | 16,613 | ||||||
| Loss from operations |
(7,784 | ) | (7,912 | ) | ||||
| Other income (expense): |
||||||||
| Amortization of deferred financing costs and debt discount |
(2,918 | ) | (2,138 | ) | ||||
| Interest expense — related party |
(747 | ) | (29 | ) | ||||
| Interest expense |
(225 | ) | (170 | ) | ||||
| Derivative fair value adjustment |
185 | 20 | ||||||
| Other income |
12 | 23 | ||||||
|
|
|
|
|
|||||
| Total other expense: |
(3,693 | ) | (2,294 | ) | ||||
|
|
|
|
|
|||||
| Net loss |
$ | (11,477 | ) | (10,206 | ) | |||
|
|
|
|
|
|||||
| Net loss per share: |
||||||||
| Basic and diluted |
$ | (1.73 | ) | $ | (2.53 | ) | ||
|
|
|
|
|
|||||
| Basic and diluted, pro forma (unaudited) |
$ | |||||||
|
|
|
|||||||
| Weighted average common share outstanding: |
||||||||
| Basic and diluted |
6,642,837 | 4,034,720 | ||||||
|
|
|
|
|
|||||
| Basic and diluted, pro forma (unaudited) |
||||||||
|
|
|
|||||||
The accompanying notes are an integral part of the financial statements.
F-4
SCYNEXIS, INC.
STATEMENTS OF CHANGES IN CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT
(in thousands)
| Series A Convertible Preferred Stock |
Series B Convertible Preferred Stock |
Series C Convertible Preferred Stock |
Series C-1 Convertible Preferred Stock |
Series C-2 Convertible Preferred Stock |
Common Stock |
Additional Paid-in Capital |
Accumulated Deficit |
Total Stockholders’ Deficit |
||||||||||||||||||||||||||||||
| (As Restated) | (As Restated) | (As Restated) | ||||||||||||||||||||||||||||||||||||
| Balance as originally reported, January 1, 2011 |
$ | 250 | $ | 6,415 | $ | 30,121 | $ | 3,200 | $ | 13,500 | $ | 4 | $ | 3,119 | $ | (59,548 | ) | $ | (56,425 | ) | ||||||||||||||||||
| Restatement to recognize the debt guarantee as deemed contribution |
— | — | — | — | — | — | 6,338 | — | 6,338 | |||||||||||||||||||||||||||||
| Restatement to record amortization of deferred financing costs |
— | — | — | — | — | — | — | $ | (1,585 | ) | (1,585 | ) | ||||||||||||||||||||||||||
| Restatement to reflect classification of warrants as derivative liability |
— | — | — | — | — | — | (256 | ) | — | (256 | ) | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance as restated, January 1, 2011 |
$ | 250 | $ | 6,415 | $ | 30,121 | $ | 3,200 | $ | 13,500 | $ | 4 | $ | 9,201 | $ | (61,133 | ) | $ | (51,928 | ) | ||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (10,206 | ) | (10,206 | ) | |||||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | — | — | 43 | — | 43 | |||||||||||||||||||||||||||||
| Exercise of warrants |
— | — | — | — | — | — | 3 | — | 3 | |||||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | 382 | — | 382 | |||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance as restated, December 31, 2011 |
$ | 250 | $ | 6,415 | $ | 30,121 | $ | 3,200 | $ | 13,500 | $ | 4 | $ | 9,629 | $ | (71,339 | ) | $ | (61,706 | ) | ||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (11,477 | ) | (11,477 | ) | |||||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | — | — | 7 | — | 7 | |||||||||||||||||||||||||||||
| Exercise of warrants |
— | — | — | — | — | — | 3 | — | 3 | |||||||||||||||||||||||||||||
| Preferred stock conversion |
— | (2,200 | ) | (2,000 | ) | (3,200 | ) | — | 3 | 7,397 | — | 7,400 | ||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | 358 | — | 358 | |||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance as restated, December 31, 2012 |
$ | 250 | $ | 4,215 | $ | 28,121 | $ | — | $ | 13,500 | $ | 7 | $ | 17,394 | $ | (82,816 | ) | $ | (65,415 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
The accompanying notes are an integral part of the financial statements.
F-5
SCYNEXIS, INC.
(in thousands)
| Year ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| (As Restated) | (As Restated) |
|||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (11,477 | ) | $ | (10,206 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Gain on sale of asset, net of transaction expenses |
(3,412 | ) | — | |||||
| Depreciation |
1,489 | 1,695 | ||||||
| Stock-based compensation expense |
358 | 382 | ||||||
| Amortization of deferred financing costs and debt discount |
2,918 | 2,138 | ||||||
| Allowance for bad debts |
(204 | ) | 450 | |||||
| Change in fair value of derivative liability |
(185 | ) | (20 | ) | ||||
| Changes in deferred rent |
(26 | ) | (1 | ) | ||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable and unbilled services |
(430 | ) | (915 | ) | ||||
| Prepaid expenses, other assets, and deferred costs |
77 | 419 | ||||||
| Accounts payable and accrued expenses |
(408 | ) | (1,306 | ) | ||||
| Interest payable — related party |
747 | 29 | ||||||
| Deferred revenue |
(43 | ) | (1,623 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(10,596 | ) | (8,958 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from investing activities: |
||||||||
| Proceeds from sale of asset, net of transaction expenses |
3,412 | — | ||||||
| Purchases of property and equipment |
(361 | ) | (276 | ) | ||||
|
|
|
|
|
|||||
| Net cash provided by (used in) investing activities |
3,051 | (276 | ) | |||||
|
|
|
|
|
|||||
| Cash flows from financing activities: |
||||||||
| Borrowings under revolving credit facility |
— | 7,000 | ||||||
| Proceeds from issuance of convertible notes and related warrants |
5,947 | 5,503 | ||||||
| Debt issuance costs |
— | (186 | ) | |||||
| Proceeds from exercise of stock options |
7 | 43 | ||||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
5,954 | 12,360 | ||||||
|
|
|
|
|
|||||
| (Decrease) increase in cash and cash equivalents |
(1,591 | ) | 3,126 | |||||
| Cash and cash equivalents, beginning of year |
3,976 | 850 | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents, end of year |
$ | 2,385 | $ | 3,976 | ||||
|
|
|
|
|
|||||
| Supplemental cash flow information: |
||||||||
| Cash paid for interest |
$ | 236 | $ | 190 | ||||
|
|
|
|
|
|||||
| Noncash financing activities: |
||||||||
| Issuance of warrants allocated to debt discount |
$ | 328 | $ | 304 | ||||
|
|
|
|
|
|||||
| Conversion of preferred shares into common shares |
$ | 7,400 | $ | — | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of the financial statements.
F-6
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
1. Description of Business and Basis of Preparation
Organization
SCYNEXIS, Inc. (“SCYNEXIS” or the “Company”) is a Delaware corporation formed on November 4, 1999. SCYNEXIS is a chemistry-focused drug discovery and development company headquartered in Research Triangle Park, North Carolina.
The Company offers its services and partnerships in the drug discovery and development phases, primarily in the form of integrated research teams consisting of medicinal, computational, analytical, and process scientists working on a collaborative basis with its customers on research projects.
Going Concern
The Company has experienced recurring losses from operations and negative cash flows due to its ongoing research and development investment in cyclophillin inhibitor and anti-fungal products. The Company also has negative working capital and debt that will become due in December 2014. The conditions described above raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The Company believes it will receive continued support from its existing investors, and it intends to raise additional funds through an initial public equity offering, the proceeds from which would enable the Company to carry on its activities and meet its obligations for at least the next 12 months. If continued support from the Company’s investors is not received or if the planned initial public offering is not successful, the Company will be required to obtain additional sources of financing through a debt or equity offering, or through the sale of assets in order to meet its obligations when they become due.
2. Summary of Significant Accounting Policies
Unaudited Pro Forma Presentation
The unaudited pro forma net loss per share for the year ended December 31, 2012 assumes the conversion of all outstanding shares of convertible preferred stock and the exercise of all common stock warrants issued with the convertible notes into an aggregate of approximately million shares of common stock upon the completion of an initial public offering (“IPO”) as of January 1, 2012 or the time of issuance, if later.
The Company believes that the unaudited pro forma net loss per share provides material information to investors because the conversion of the convertible preferred stock into common stock and the exercise of common stock warrants issued with the convertible notes are expected to occur upon the closing of an IPO and, therefore, the disclosure provides a measure of net loss per share that is comparable to what will be reported as a public company.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires the Company to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of
F-7
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates include the accounts receivable allowance, valuation of the related party deemed contribution, the fair value of the Company’s common stock used to measure stock-based compensation for options granted to employees and nonemployees and the fair value of the Company’s derivative liability.
Concentration of Credit Risk
Financial instruments, which potentially expose the Company to concentrations of credit risk, consist principally of cash on deposit with a bank, which exceeds insured limits, and accounts receivable. Ongoing credit evaluations of customer’s financial condition are performed by the Company and collateral is not required.
One customer represented 18% of accounts receivable at December 31, 2012, and another customer represented 56% of accounts receivable at December 31, 2011.
The following customers accounted for 10% or more of the Company’s revenues in the years ended December 31, 2012 and 2011:
| Year Ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| Customer A |
44 | % | 51 | % | ||||
| Customer B |
— | 13 | % | |||||
| Customer C |
— | 11 | % | |||||
Revenue from a stockholder of the Company accounted for 44% and 51% of revenues in 2012 and 2011 (Note 14), respectively, and are included above.
Cash and Cash Equivalents
The Company considers any highly liquid investments with a remaining maturity of three months or less when purchased to be cash and cash equivalents.
Accounts Receivable and Unbilled Services
Accounts receivable and unbilled services consist of amounts billed and unbilled under the Company’s service contracts with its customers. The Company extends credit to customers without requiring collateral. Accounts receivable are stated at net realizable value. On a periodic basis, the Company evaluates its accounts receivable and establishes an allowance based on its history of collections and write-offs and the current status of all receivables. The Company does not accrue interest on trade receivables.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation. Depreciation is determined on a straight-line basis over the estimated useful lives of the assets, which generally range from three to seven years. Leasehold improvements are amortized over the shorter of the useful life of the asset or the term of the related lease. Maintenance and repairs are charged against expense as incurred.
F-8
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
Deferred financing costs
Deferred financing costs are transaction costs associated with issuing debt as well as costs related to a deemed contribution for a guarantee from a related party (see Note 18). The Company recognizes these costs in the balance sheet as noncurrent assets. Deferred financing costs are amortized over the life of the related debt.
Other Assets
Other assets consist primarily of the refundable long-term deposit on the leased building facility and of the refundable amount held by the Company’s employee dental plan insurance provider as required by its agreement.
Impairment of Long-Lived Assets
Long-lived assets to be held and used are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. When such events occur, the Company compares the carrying amounts of the assets to their undiscounted expected future cash flows. If the undiscounted cash flows are insufficient to recover the carrying value, an impairment loss is recorded for the difference between the carrying value and fair value of the asset. To date, no such impairment has occurred.
Revenue Recognition and Deferred Revenue
The Company derives the majority of its revenue from providing contract research and development services under fee for service arrangements. The Company also has entered into collaboration arrangements in exchange for non-refundable upfront payments and consideration as services are performed. These arrangements include multiple elements, such as the sale of licenses and the provision of services. Under these arrangements, the Company also is entitled to receive development milestone payments and royalties in the form of a designated percentage of product sales.
Revenue is recognized when all of the following conditions are met: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) fees are fixed or determinable, and (iv) collection of fees is reasonably assured.
The Company’s contract research and development services revenue is recognized in the period in which the services are performed. The Company historically has recognized milestone payments received on a straight-line basis over the remaining service period. No milestone payments were received in either period presented in the accompanying statements of operations. License revenue in the form of upfront payments is deferred and recognized over the applicable relationship period. The Company recognized an immaterial amount of license revenue from the receipt of upfront payments in the accompanying statement of operations. The Company has not received any royalty payments to date.
Amounts received prior to satisfying all revenue recognition criteria are recorded as deferred revenue in the accompanying balance sheets.
Fair Value of Financial Instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date, based on the Company’s principal or, in absence of a principal, most advantageous market for the specific asset or liability.
F-9
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
The Company uses a three-tier fair value hierarchy to classify and disclose all assets and liabilities measured at fair value on a recurring basis, as well as assets and liabilities measured at fair value on a non-recurring basis, in periods subsequent to their initial measurement. The hierarchy requires the Company to use observable inputs when available, and to minimize the use of unobservable inputs when determining fair value. The three tiers are defined as follows:
| Ÿ | Level 1 — Observable inputs that reflect quoted market prices (unadjusted) for identical assets or liabilities in active markets; |
| Ÿ | Level 2 — Observable inputs other than quoted prices in active markets that are observable either directly or indirectly in the marketplace for identical or similar assets and liabilities; and |
| Ÿ | Level 3 — Unobservable inputs that are supported by little or no market data, which require the Company to develop its own assumptions about the assumptions market participants would use in pricing the asset or liability based on the best information available in the circumstances. |
Research and Development
Major components of research and development costs include cash compensation, stock-based compensation, preclinical studies, clinical trial and related clinical manufacturing, drug development, materials and supplies, legal, regulatory compliance, and fees paid to consultants and other entities that conduct certain research and development activities on the Company’s behalf.
Amortization of Deferred Financing Costs and Debt Discount
Amortization of deferred financing costs and debt discount includes the amortization of debt discount related to the warrants issued with the convertible notes, the amortization of issuance costs related to the convertible notes, and amortization of the deferred financing costs related to a deemed contribution for a guarantee from a related party (see Note 18).
Comprehensive Loss
The Company has no items of comprehensive income or loss other than net loss.
Income Taxes
The Company provides for deferred income taxes under the asset and liability method, whereby deferred income taxes result from temporary differences between the tax bases of assets and liabilities and their reported amounts in the financial statements. Valuation allowances are established when necessary to reduce deferred tax assets to the amount that the Company believes is more likely than not to be realized. The Company recognizes uncertain tax positions when the positions will be more likely than not sustained based solely upon the technical merits of the positions.
Stock-Based Compensation
The Company measures and recognizes compensation expense for all stock-based payment awards made to employees, officers, and directors based on the estimated fair values of the awards as of grant date. The value of the portion of the award that is ultimately expected to vest is recorded as expense over the requisite service periods.
F-10
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
The Company also accounts for equity instruments issued to non-employees using a fair value approach. The Company values equity instruments, stock options and warrants granted to lenders and consultants using the Black-Scholes valuation model. The measurement of non-employee share-based compensation is subject to periodic adjustments as the underlying equity instruments vest and is recognized as an expense over the term of the related financing or the period over which services are received.
Deferred Rent
The Company recognizes rent expense on a straight-line basis over the non-cancelable term of its operating lease and records the difference between cash rent payments and the recognition of rent expense as a deferred rent liability. The Company also records landlord-funded lease incentives, such as reimbursable leasehold improvements, as a deferred rent liability, which is amortized as a reduction of rent expense over the non-cancelable term of its operating lease.
Basic and Diluted Net Loss per Share of Common Stock
The Company uses the two-class method to compute net loss per common share because the Company has issued securities, other than common stock, that contractually entitle the holders to participate in dividends and earnings of the Company. The two-class method requires earnings for the period to be allocated between common stock and participating securities based upon their respective rights to receive distributed and undistributed earnings. Holders of each series of the Company’s convertible preferred stock are entitled to participate in distributions, when and if declared by the board of directors that are made to common stockholders, and as a result are considered participating securities.
Segment and Geographic Information
Operating segments are defined as components of an enterprise (business activity from which it earns revenue and incurs expenses) about which discrete financial information is available and regularly reviewed by the chief operating decision maker in deciding how to allocate resources and in assessing performance. The Company’s chief operating decision maker (“CODM”) is the Chief Executive Officer. The CODM reviews consolidated operating results to make decisions about allocating resources and assessing performance for the entire Company. The Company views its operations and manages its business as one operating segment. All assets of the Company were held in the United States for the years ended December 31, 2012 and 2011.
Although all operations are based in the United States, the Company generated a portion of its revenue from customers outside of the United States. Information about the Company’s revenue from different geographic regions for the year ended December 31, 2012 and 2011 is presented as follows:
| Year Ended December 31, | ||||||||||||||||
| 2012 | 2011 | |||||||||||||||
| United States |
$ | 13,072 | 78 | % | $ | 23,311 | 88 | % | ||||||||
| Europe |
3,765 | 22 | % | 3,143 | 12 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 16,837 | 100 | % | $ | 26,454 | 100 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
All sales, including sales outside of the United States, are denominated in the United States dollar.
F-11
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
3. Allowance for Bad Debts
Summary of activity in the allowance for bad debts for the years ended December 31, 2012 and 2011 was the following:
| Balance at Beginning of Period |
Additions Charged to Expense |
Deductions | Balance at End of Period |
|||||||||||||
| Allowance for bad debts: |
||||||||||||||||
| Year ended December 31, 2011 |
$ | 5 | $ | 455 | $ | (5 | ) | $ | 455 | |||||||
| Year ended December 31, 2012 |
$ | 455 | $ | 50 | $ | (254 | ) | $ | 251 | |||||||
4. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist of the following:
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Prepaid service contract |
$ | 129 | $ | 193 | ||||
| Prepaid insurance |
83 | 70 | ||||||
| Other prepaid expenses |
201 | 128 | ||||||
| Other current assets |
8 | 89 | ||||||
|
|
|
|
|
|||||
| $ | 421 | $ | 480 | |||||
|
|
|
|
|
|||||
5. Property and Equipment
Property and equipment consists of the following:
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Equipment |
$ | 9,665 | $ | 10,327 | ||||
| Furniture and fixtures |
399 | 427 | ||||||
| Leasehold improvements |
13,115 | 13,055 | ||||||
|
|
|
|
|
|||||
| Total property and equipment |
23,179 | 23,809 | ||||||
| Less accumulated depreciation |
(16,895 | ) | (16,397 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment — net |
$ | 6,284 | $ | 7,412 | ||||
|
|
|
|
|
|||||
Depreciation expense for the years ended December 31, 2012 and 2011 was $1,489 and $1,695, respectively.
6. Debt Obligations
Credit Facility Agreement
In April 2010, the Company entered into a $15,000 credit facility agreement with HSBC bank (the “2010 Credit Agreement”). The agreement comprises a $5,000 term loan and a $10,000 revolving credit facility. Borrowings under the 2010 Credit Agreement carry interest at a rate of London InterBank Offered
F-12
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
Rate plus 0.95% per annum. The weighted-average interest rate was 1.4% and 1.3% for the years ended December 31, 2012 and 2011, respectively. As of December 31, 2012 and 2011, both the $5,000 term loan and the $10,000 revolving credit facility were outstanding. The 2010 Credit Agreement required interest-only payments through March 2013. All outstanding borrowings under the agreement were due on March 11, 2013. As described in Note 19, the terms of the 2010 Credit were amended in March 2013 to extend the due date to December 31, 2014. The 2010 Credit Agreement is guaranteed by a related party that has an investment in the Company. The 2010 Credit Agreement contains no financial covenants.
As discussed in Note 18, the Company has concluded that a deemed contribution in relation to the guarantee by the related party should be recognized at the inception of the 2010 Credit Agreement.
Note and Warrant Purchase Agreement
In December 2011, the Company issued convertible notes and warrants to related parties that hold investments in the Company and received $5,503. The total principal amount of the convertible notes is $5,500 and the convertible notes bear interest at a rate of 8% per annum. On January 27, 2012 and May 15, 2012, the Company received $222 and $5,725, respectively, from the issuance of additional convertible notes and warrants under the same agreement. The total principal amount of the convertible notes is $11,444 and the convertible notes bear interest at a rate of 8% per annum. The outstanding principal amount of the convertible notes and unpaid accrued interest is due on December 31, 2012, contingent upon (i) the prior written consent of holders of at least 70% of the outstanding aggregated principal amount of the convertible notes and (ii) the prior written consent of HSBC Bank for so long as any of the principal and interest related to the 2010 Credit Agreement remains outstanding. These events did not occur as of December 31, 2012, and thus, the convertible notes were outstanding as of December 31, 2012. The convertible notes contain no financial covenants.
These convertible notes are convertible into shares of the Company’s stock through different methods, including:
| Ÿ | In the event the Company issues and sells shares of its equity securities to investors on or before June 30, 2012, in an equity financing with total proceeds actually received by the Company of not less than $25,000 including the conversion of the aggregate principal amount and all unpaid accrued interest outstanding under the convertible notes (a “Qualified Financing”), the outstanding principal balance of the convertible notes shall automatically convert in whole without any further action by the noteholders into such equity securities at a price equal to 85% of the issue price of such equity securities. Equity securities shall mean any series of preferred stock that (i) ranks pari passu or senior to the Company’s Series C-2 Convertible Preferred Stock upon any liquidation, dissolution or winding-up of the Company and upon any acquisition or asset transfer and (ii) is convertible into shares of common stock of the Company. This conversion option is no longer available given it expired on June 30, 2012. |
| Ÿ | Upon the occurrence of either an acquisition or asset transfer, the entire outstanding principal balance of the convertible notes shall at the option of the noteholder either (i) become fully due and payable, provided however that the repayment shall also require prior written consent of the noteholder majority and HSBC Bank or (ii) convert into whole shares of the Company’s Series D-1 or Series D-2 Preferred Stock as applicable at a conversion price equal to $4.3125 per share subject to proportionate and equitable adjustment upon any stock split, stock dividend, reverse stock split or other similar event. |
F-13
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
| Ÿ | Upon closing by the Company of any equity financing that is not a Qualified Financing, the entire principal balance of the convertible notes and all unpaid accrued interest shall at the sole option of the noteholder convert in whole into the same class or type of equity securities sold by the Company in connection with such equity financing. The conversion price shall be at a conversion price that is equal to the price paid by the investors participating in such equity financing and shall otherwise be on the same terms and conditions applicable to such investors. |
| Ÿ | Upon written consent of the Company and noteholder majority, the aggregate principal balance of the convertible notes and all accrued interest shall be automatically converted into shares of the Company’s Series D-1 Preferred stock or Series D-2 Preferred stock as applicable pursuant to the conversion price detailed above at any time on or after December 31, 2012. |
None of the events that trigger conversion of the convertible notes occurred during the year ended December 31, 2012. Total notes payable due as of December 31, 2012 and 2011 were classified as current and amounted to $11,444 and $5,215, respectively.
As described in Note 19, in December 2013 the convertible noteholders elected to convert the outstanding principal and accrued interest under the Notes into Series D-1 Preferred and Series D-2 Preferred.
A warrant to purchase common stock of the Company was issued to each noteholder. The fair value at the date of issuance for the warrants issued in 2012 and 2011 was $328 and $304, respectively. The warrant fair values were accounted for as debt discount and were amortized to interest expense over the stated term of the convertible notes. The amount of discount amortization related to the warrant issuances recorded as expense in the statement of operations for the years ended December 31, 2012 and 2011 was $613 and $19, respectively. As of December 31, 2012 and 2011, the discount on the convertible notes related to the warrant issuances was $0 and $285, respectively. The Company determined that the warrants should be recorded as a derivative liability and stated at fair value at each reporting period (see Note 17).
Future Debt Maturities
Future debt maturities as of December 31, 2012, are as follows:
| 2013 |
$ | 11,444 | ||
| 2014 |
15,000 | |||
|
|
|
|||
| Total |
$ | 26,444 | ||
|
|
|
7. Accrued Expenses
Accrued expenses consist of the following:
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Accrued research and development expenses |
$ | 440 | $ | 952 | ||||
| Other accrued expenses |
341 | 569 | ||||||
| Interest payable |
30 | 41 | ||||||
|
|
|
|
|
|||||
| $ | 811 | $ | 1,562 | |||||
|
|
|
|
|
|||||
F-14
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
8. Commitments and Contingencies
Leases
The Company leases its facilities and certain office equipment under long-term non-cancelable operating leases. The Company’s lease for its primary North Carolina facility expires in 2014. The lease has two optional five-year renewal periods through 2024. The first optional renewal period for the original lease space has been included in the future minimum lease payments, as the Company would incur a significant economic penalty through relocation or replacement of leasehold improvements prior to the end of their useful lives.
Rent expense was approximately $1,049 and $1,346 for the years ended December 31, 2012 and 2011, respectively. Future minimum lease payments for all operating expenses as of December 31, 2012 are as follows:
| 2013 |
$ | 919 | ||
| 2014 |
1,049 | |||
| 2015 |
1,104 | |||
| 2016 |
1,137 | |||
| 2017 |
1,171 | |||
| Thereafter |
1,510 | |||
|
|
|
|||
| Total |
$ | 6,890 | ||
|
|
|
Contingencies
A former client has alleged that the Company breached its service agreement with the former client and has requested that the Company pay $443 in compensation. This matter is currently within the federal court system and in the discovery phase. The Company believes the claim is without merit and the Company intends to vigorously defend itself. The Company is unable to predict the outcome of the matter, but does not believe it will result in a material impact on the Company’s financial position, results of operations, or cash flows. See Note 19 for the resolution of this matter.
9. Convertible Preferred Stock
Convertible Preferred stock has par value $0.001 and was issued beginning in 2000. Each issuance is briefly described as follows:
Series A Convertible Preferred Stock (“Series A Preferred”)
In 2000, the Company issued 31,407 shares of Series A Preferred at $7.96 per share to initial employees and consultants of SCYNEXIS.
Series B Convertible Preferred Stock (“Series B Preferred”)
In 2000, the Company issued 600,999 shares of Series B Preferred at $9.01 per share in exchange for $2,200 in equipment, intellectual property, and conversion of existing debt, and $3,215 in cash and incurred issuance costs of $43. Subsequently in 2000, the Company issued an additional 110,988 shares of Series B convertible preferred stock at $9.01 per share for cash. As part of the issuance of the Series C convertible
F-15
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
preferred stock in June 2002, the holders of Series B Preferred agreed to modify the redemption feature of the Series B Preferred to eliminate the redemption feature of the Series B Preferred. As described below, 244,173 shares of Series B Preferred were mandatorily converted to common stock during 2012.
Series C Convertible Preferred Stock (“Series C Preferred”) and Warrants
The Company issued warrants to purchase 100,524 shares of Series C Preferred in conjunction with certain bridge loan financings during 2001 and the subsequent 2002 Series C Preferred financing. The warrants were issued with an exercise price of $0.01 per share. Two of the investors exercised such warrants during 2003.
In 2002, the Company issued 2,867,154 shares of Series C Preferred for $24,000 in cash and the conversion of approximately $4,513 of 4.5% convertible notes and accrued interest, less issuance costs of approximately $86. As described below, 197,045 shares of Series C Preferred were mandatorily converted to common stock during 2012. In January 2005, the remaining warrants to purchase 23,911 shares of Series C Preferred shares were fully exercised.
Series C-1 Convertible Preferred Stock (“Series C-1 Preferred”) and Warrants
In August 2004, the Company received cash of $3,200 for the issuance of 984,615 shares of Series C-1 Preferred. As described below, these Series C-1 Preferred shares were mandatorily converted to common stock during 2012.
In July 2006, the Company issued warrants to purchase 196,923 shares of Series C-1 Preferred in conjunction with a loan financing agreement. The warrants were issued with an exercise price of $3.25 per share and expire on July 14, 2016. The fair value at the date of grant for these instruments was $459, which was recorded as a debt discount. The debt discount related to these warrants was fully amortized as of December 31, 2010. The Company determined that the warrants should be recorded as a derivative liability and stated at fair value at each reporting period (see Note 18). The Company recorded other income of $79 and $20 for the years ended December 31, 2012 and 2011, respectively, related to the fair value adjustment for these warrants.
Series C-2 Convertible Preferred Stock (“Series C-2 Preferred”)
In March 2008, the Company received cash of $13,500 for the issuance of 2,347,826 shares of Series C-2 Preferred.
Series D-1 Convertible Preferred Stock (“Series D-1 Preferred) and Series D-2 Convertible Preferred Stock (“Series D-2 Preferred”)
Shares of Series D-1 Preferred and Series D-2 Preferred (together “Series D Preferred”) are authorized, but none are issued or outstanding as of December 31, 2012 and 2011.
F-16
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
Authorized, Issued, and Outstanding Preferred Shares
The following table summarizes authorized, issued and outstanding preferred shares as of December 31, 2012:
| Authorized | Outstanding | Issue Price | Liquidation Preference |
|||||||||||||
| Series A Preferred |
31,410 | 31,407 | $ | 7.96 | $ | 250 | ||||||||||
| Series B Preferred |
711,987 | 467,814 | 9.01 | 4,215 | ||||||||||||
| Series C Preferred |
2,967,678 | 2,770,633 | 10.15 | 28,121 | ||||||||||||
| Series C-1 Preferred |
3,076,923 | — | 3.25 | — | ||||||||||||
| Series C-2 Preferred |
2,347,826 | 2,347,826 | 5.75 | 13,500 | ||||||||||||
| Series D-1 Preferred |
5,000,000 | — | 4.31 | — | ||||||||||||
| Series D-2 Preferred |
5,000,000 | — | 4.31 | — | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total |
19,135,824 | 5,617,680 | $ | 46,086 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
Preferred Stock Activity
The following table summarizes preferred stock activity for the years ended December 31, 2012 and 2011:
| Shares of | ||||||||||||||||||||
| Series A Convertible Preferred Stock |
Series B Convertible Preferred Stock |
Series C Convertible Preferred Stock |
Series C-1 Convertible Preferred Stock |
Series C-2 Convertible Preferred Stock |
||||||||||||||||
| Balance, January 1, 2011 |
31,407 | 711,987 | 2,967,678 | 984,615 | 2,347,826 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance, December 31, 2011 |
31,407 | 711,987 | 2,967,678 | 984,615 | 2,347,826 | |||||||||||||||
| Conversion into common stock |
— | (244,173 | ) | (197,045 | ) | (984,615 | ) | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance, December 31, 2012 |
31,407 | 467,814 | 2,770,633 | — | 2,347,826 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Significant terms of the convertible preferred stock are as follows:
Voting rights
Each share has the right to vote equal to the number of shares of common stock into which it is convertible. Additionally, the approval of 65% of the Series B Preferred, Series C Preferred, and Series C-2 Preferred stockholders, voting as separate classes, is required to change any bylaws; issue stock or securities with a preference to Series B Preferred, Series C Preferred, and Series C-2 Preferred; change any rights, preferences and privileges of Series B Preferred, Series C Preferred, and Series C-2 Preferred; or change the number of directors outside a range. Furthermore, the approval of 65% of the Series C Preferred stockholders is required to liquidate, sell, or merge the Company.
Approval of 70% of the Series D Preferred stockholders, voting as a separate class, is required to change any bylaws; issue stock or securities with a preference to Series D Preferred; or change any rights, preferences and privileges of Series D Preferred.
F-17
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
Dividend rights
Holders of Series D Preferred are entitled to receive 8% of the original issue price per annum as a dividend on a “when and if” declared basis in preference to any dividend paid to other convertible preferred or common stockholders. Such dividends are payable only when, and if, declared by the Board of Directors and are noncumulative.
After payment of the 8% Series D dividend, holders of all series of convertible preferred stock are entitled to receive dividends declared by the Board of Directors in preference to any dividend paid to common stockholders. Each share of preferred stock is entitled to the same amount as would have been declared or paid thereon had the holder thereof elected to convert the same into shares of common stock.
Holders of Series D-1 Preferred and Series D-2 Preferred have a liquidation preference of two and three times the original issue price plus all declared and unpaid dividends adjusted for events of dilution, respectively. Holders of Series A Preferred, Series B Preferred, Series C Preferred, Series C-1 Preferred, and Series C-2 Preferred have liquidation preferences of $7.96, $9.01, $10.15, $3.25, and $5.75 per share, plus declared but unpaid dividends adjusted for events of dilution, respectively. Upon occurrence of a liquidation event, Series D-1 Preferred and Series D-2 participate pari passu; then Series C-2 Preferred, Series C-1 Preferred, and Series C Preferred participate pari passu; then Series B Preferred; then Series A Preferred would receive their liquidation preference; and the remaining assets would be distributed ratably to the preferred and common stockholders on an “as converted” basis.
Conversion rights
Each share of Series A Preferred, Series B Preferred, and Series C Preferred is convertible into four shares of common stock, subject to adjustment for events of dilution, at the option of the holder any time after the date of issuance.
Each share of Series C-1 Preferred, Series C-2 Preferred, Series D-1 Preferred, and Series D-2 Preferred is convertible into one share of common stock, subject to adjustment for events of dilution, at the option of the holder any time after the date of issuance.
Series A Preferred will convert automatically into common stock at the conversion price upon completion of a public offering of the common stock of the Company.
Shares of Series B Preferred will convert automatically into common stock at the conversion price upon completion of a public offering of common stock with aggregate proceeds not less than $15,000 and at a price per share of not less than $9.01.
Shares of Series C Preferred, Series C-1 Preferred, and Series C-2 Preferred will convert automatically into common stock at the conversion price upon completion of a public offering of common stock with aggregate proceeds not less than $30,000 and at a price per share of not less than $11.00.
Shares of Series D Preferred will convert automatically into common stock at the conversion price upon completion of a public offering of common stock with aggregate proceeds not less than $30,000 and the public offering yields an IPO pre-money value of at least $250,000.
The conversion price for Series B Preferred, Series C Preferred, Series C-1 Preferred, Series C-2 Preferred, and Series D Preferred are subject to adjustment if the Company issues additional shares of
F-18
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
common stock at a price less than the Series B Preferred, Series C Preferred, Series C-1 Preferred, Series C-2 Preferred, and Series D Preferred conversion prices in effect at the time of the sale.
In conjunction with the issuance of the convertible note with attached warrants, the Company implemented a special mandatory conversion provision. Under this provision, preferred stockholders that met certain ownership criteria who elected not to purchase their pro rata amount of the convertible note round had their preferred shares converted into common stock in 2012.
Redemption
Upon liquidation, dissolution, or winding up of the Company, the holders of the Series D-2 Preferred receive an amount equal to three times the original issue price plus all declared and unpaid dividends; the holders of the Series D-1 Preferred receive an amount equal to two times the original issue price plus all declared and unpaid dividends; and the holders of the Series C-2 Preferred, Series C-1 Preferred, Series C Preferred, Series B Preferred, and the Series A Preferred receive an amount equal to the original issue price plus all declared and unpaid dividends. In addition, after receiving their liquidation preference, the holders of all series of preferred stock share ratably with holders of common stock on an as-if-converted to common stock basis. An asset transfer or acquisition of the Company is a deemed liquidation event in that holders of all series of preferred stock are treated in the same manner as upon liquidation, dissolution, or winding up of the Company. As a result of the existence of this deemed liquidation feature, the Company determined that all series of preferred stock are redeemable. They are carried at liquidation value at each reporting period and excluded from stockholders’ deficit in the accompanying balance sheets.
10. Common Stock
Authorized, Issued and Outstanding Common Shares
The Company’s common stock has a par value of $0.001 per share and consists of 54,000,000 authorized shares at December 31, 2012 and 2011, respectively, and 6,851,149 and 4,067,347 shares issued and outstanding at December 31, 2012 and 2011, respectively. At December 31, 2012, the Company had reserved a total of 23,190,670 of its authorized 54,000,000 shares of common stock for future issuance as follows:
| For conversion of Series A Preferred, Series B Preferred, Series C Preferred, and Series C-2 Preferred and exercise of warrants to purchase Series C-1 Preferred and subsequent conversion of the shares purchased |
15,987,765 | |||
| Outstanding stock options |
3,149,271 | |||
| Outstanding common stock warrants |
543,027 | |||
| For possible conversion of notes and interest payable |
2,833,566 | |||
| For possible future issuance under stock option plan |
677,041 | |||
|
|
|
|||
| Total common shares reserved for future issuance |
23,190,670 | |||
|
|
|
F-19
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
Common Stock Activity
The following table summarizes common stock shares activity for the years ended December 31, 2012 and 2011:
| Shares of Common Stock |
||||
| Balance, January 1, 2011 |
3,981,947 | |||
| Exercise of stock options |
85,400 | |||
|
|
|
|||
| Balance, December 31, 2011 |
4,067,347 | |||
| Exercise of stock options |
6,685 | |||
| Preferred stock conversion |
2,777,117 | |||
|
|
|
|||
| Balance, December 31, 2012 |
6,851,149 | |||
|
|
|
|||
Liquidation Rights
In the event of any liquidation or dissolution of the Company, the holders of the common stock are entitled to the remaining assets of the Company legally available for distribution after the payment of the full liquidation preference for all series of the outstanding preferred stock.
Dividends and Voting Rights
The holders of the common stock are entitled to receive dividends if and when declared by the Company, but not until all dividends on the preferred stock have been either (i) paid or (ii) declared and the Company has set aside the funds to pay those dividends declared. The holders of the common stock have the right to one vote per share.
Warrants
In 2005 and 2004, in connection with the procurement of a debt financing agreement used to purchase equipment during those years, the Company issued warrants to purchase 4,253 and 3,267 shares of common stock, respectively. The warrants were issued with an exercise price of $2.54 per share and expired on June 28, 2011. The fair value at the date of grant for these instruments was insignificant.
In 2007, in connection with the procurement of a debt financing agreement used to purchase equipment during that year, the Company issued warrants to purchase 12,308 shares of common stock. The warrants were issued with an exercise price of $3.25 per share and will expire on September 14, 2014. The fair value at the date of grant for these instruments was insignificant.
In 2012 and 2011, in connection with the issuance of convertible notes, the Company issued warrants to purchase 530,719 shares of common stock (see Note 6 for disclosures regarding the convertible notes). The warrants may be exercised into common stock at the earliest of:
| (i) | the date the related convertible notes are converted in accordance with the terms above, |
| (ii) | the date the related convertible notes are repaid or prepaid in full in accordance with the terms above, and |
F-20
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
| (iii) | June 30, 2012. |
No warrants were exercised during 2012 or 2011. These warrants will expire on June 30, 2017. The exercise price of the warrants is $0.01 per share of common stock and the number of shares of common stock that may be purchased by exercising the warrants is calculated as follows:
| Ÿ | If a related convertible note is converted pursuant to and in accordance with the terms above, then the number of shares of common stock shall be equal to the quotient of (A) the product of (i) 20% multiplied by (ii) the principal amount of the convertible note divided by (B) the applicable per share conversion price at which the related convertible note is so converted; or |
| Ÿ | If a related convertible note is repaid or prepaid in full prior to the conversion thereof pursuant to and in accordance with the terms above, then the number of shares of common stock shall be equal to the quotient of (A) the product of (i) 20% multiplied by (ii) the principal amount of the convertible note divided by (B) $4.3125 (subject to proportionate adjustment upon any adjustment to the Series D-1 Preferred or Series D-2 Preferred conversion price as defined in the Company’s Fourth Amended and Restated Certificate of Incorporation); or |
| Ÿ | If a warrant is first exercised at any time after June 30, 2012, and such first exercise of the warrant occurs prior to the conversion, repayment or prepayment of the related convertible note pursuant to and in accordance with the terms above, then the number of shares of common stock shall be equal to the quotient of (A) the product of (i) 20% multiplied by (ii) the principal amount of the convertible note divided by (B) $4.3125 (subject to proportionate adjustment upon any adjustment to the Series D-1 Preferred or Series D-2 Preferred conversion price as defined in the Company’s Fourth Amended and Restated Certificate of Incorporation). |
These warrants meet the definition of a derivative financial instrument and are accounted for as derivatives. The combined fair values of the common stock warrant derivative liabilities is $525 and $304 as of December 31, 2012 and 2011, respectively, and is recorded as a long-term derivative liability in the balance sheet. The Company recorded other income of $106 and $0 for the years ended December 31, 2012 and 2011, respectively, related to the fair value adjustment of the long-term derivative liability for common stock warrants.
11. Stock-based Compensation
The Company has a share-based compensation plan. The compensation cost that has been charged against income for this plan for the years ended December 31, 2012 and 2011 was $358 and $382, respectively. The total income tax benefit recognized in the statements of operations for share-based compensation arrangements was $0 for 2012 and 2011. Cash received or receivable from options exercised was $7 and $43 for the years ended December 31, 2012 and 2011, respectively.
Under the Company’s stock option plan, the Company may grant options to purchase up to approximately 4,126,000 shares of common stock to employees, directors, and consultants as either incentive stock options or nonqualified stock options. Incentive stock options may be granted with exercise prices not less than 100% to 110% of the fair market value of the common stock. Options granted under the plan generally vest over three to four years and expire in 10 years from the date of grant.
F-21
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
Stock-based compensation expense related to stock options is included in the following line items in the accompanying statements of operations:
| Year Ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| Cost of revenue |
$ | 103 | $ | 116 | ||||
| Research and development |
40 | 47 | ||||||
| Selling, general and administrative |
215 | 219 | ||||||
|
|
|
|
|
|||||
| $ | 358 | $ | 382 | |||||
|
|
|
|
|
|||||
The fair value of a stock option is estimated using an option-pricing model that takes into account as of the grant date the exercise price and expected life of the option, the current price of the underlying stock and its expected volatility, expected dividends on the stock, and the risk-free interest rate for the expected term of the option. The Company has used the simplified method in calculating the expected term of all option grants based on the vesting period. Compensation costs related to share-based payment transactions are recognized in the financial statements upon satisfaction of the requisite service or vesting requirements. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company based its estimated forfeiture rate on historical forfeitures of all stock option grants.
The Company has elected to use the Black-Scholes option-pricing model. The Black-Scholes option-pricing model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable rather than for use in estimating the fair value of stock options subject to vesting and transferability restrictions. Using the Black-Scholes option valuation model, the weighted-average fair value of options granted during 2012 and 2011 was $0.68 and $1.05 per option, respectively. The total fair value of options granted during 2012 and 2011 was $277 and $470, respectively. The assumptions used in these models to estimate fair value and the resulting grant date fair values are as follows:
| Employees | Nonemployees | |||||||||||||||
| Year Ended December 31, | Year Ended December 31, | |||||||||||||||
| 2012 | 2011 | 2012 | 2011 | |||||||||||||
| Expected dividend yield |
— | — | — | — | ||||||||||||
| Expected volatility |
64.10 | % | 81.79 | % | 64.10 | % | 81.79 | % | ||||||||
| Risk-free interest rate |
0.98 — 1.28 | % | 1.64 — 2.79 | % | 0.98 — 1.28 | % | 2.79 | % | ||||||||
| Expected term (in years) |
6.13 — 6.49 | 5.42 — 6.49 | 5.00 | 5.00 | ||||||||||||
| Forfeiture rate |
5.00 | % | 5.00 | % | 5.00 | % | 5.00 | % | ||||||||
F-22
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
The activity of the plan for the years ended December 31, 2012 and 2011, is summarized as follows:
| Number of Shares |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Life (in years) |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding — January 1, 2011 |
3,544,275 | $ | 1.03 | 5.27 | $ | 866 | ||||||||||
|
|
|
|||||||||||||||
| Granted |
448,400 | 1.50 | ||||||||||||||
| Exercised |
(85,400 | ) | 0.24 | |||||||||||||
| Canceled |
(141,409 | ) | 0.68 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding — December 31, 2011 |
3,765,866 | $ | 1.11 | 5.05 | $ | 1,458 | ||||||||||
|
|
|
|
|
|||||||||||||
| Exercisable — December 31, 2011 |
2,992,655 | $ | 1.04 | 4.12 | $ | 1,364 | ||||||||||
|
|
|
|
|
|||||||||||||
| Vested or expected to vest — December 31, 2011 |
3,727,205 | $ | 1.07 | 4.79 | $ | 1,453 | ||||||||||
|
|
|
|
|
|||||||||||||
| Outstanding — December 31, 2011 |
3,765,866 | $ | 1.11 | 5.05 | $ | 1,458 | ||||||||||
|
|
|
|||||||||||||||
| Granted |
405,238 | 1.20 | ||||||||||||||
| Exercised |
(6,685 | ) | 1.02 | |||||||||||||
| Canceled |
(1,015,148 | ) | 1.09 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding — December 31, 2012 |
3,149,271 | $ | 1.13 | 5.02 | $ | 213 | ||||||||||
|
|
|
|
|
|||||||||||||
| Exercisable — December 31, 2012 |
2,574,349 | $ | 1.10 | 4.14 | $ | 262 | ||||||||||
|
|
|
|
|
|||||||||||||
| Vested or expected to vest — December 31, 2012 |
3,120,525 | $ | 1.07 | 4.47 | $ | 218 | ||||||||||
|
|
|
|
|
|||||||||||||
The intrinsic values in the table above represent the total intrinsic value (the difference between the Company’s estimated fair value of common stock as of December 31, 2012 and 2011, and the exercise price multiplied by the number of options). The intrinsic value amounts presented above can be positive or negative based on the average exercise price being greater or less than the estimated fair value of common stock as of December 31, 2012 and 2011.
F-23
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
Information as of December 31, 2012, concerning currently outstanding and vested options is as follows:
| Outstanding | Exercisable | |||||||||||||||
| Exercise |
Number of Shares |
Weighted- Average Remaining Contractual Life (Years) |
Number of Shares |
Weighted- Average Remaining Contractual Life (Years) |
||||||||||||
| $1.00 |
1,767,596 | 2.64 | 1,767,596 | 2.64 | ||||||||||||
| 1.20 |
405,238 | 9.69 | 78,038 | 9.53 | ||||||||||||
| 1.25 |
337,800 | 6.31 | 335,904 | 6.31 | ||||||||||||
| 1.27 |
297,500 | 7.53 | 186,250 | 7.53 | ||||||||||||
| 1.50 |
341,137 | 8.31 | 206,561 | 8.31 | ||||||||||||
|
|
|
|
|
|||||||||||||
| 3,149,271 | 5.02 | 2,574,349 | 4.14 | |||||||||||||
|
|
|
|
|
|||||||||||||
The total fair value of shares vested during the years ended December 31, 2012 and 2011 was $716 and $469, respectively.
As of December 31, 2012 and 2011, the Company had 0 and 1,861 unvested shares, respectively, with an exercise price of $1.00. As of December 31, 2012 and 2011, the Company had 327,200 and 0 unvested shares, respectively, with an exercise price of $1.20. As of December 31, 2012 and 2011, the Company had 1,896 and 164,191 unvested shares, respectively, with an exercise price of $1.25. As of December 31, 2012 and 2011, the Company had 111,250 and 224,875 unvested shares, respectively, with an exercise price of $1.27. As of December 31, 2012 and 2011, the Company had 134,575 and 382,284 unvested shares, respectively, with an exercise price of $1.50.
As of December 31, 2012 and 2011, there was approximately $316 and $411, respectively, of total unrecognized compensation cost related to unvested share-based compensation arrangements granted under the plan. That cost is expected to be recognized over a weighted-average period of 1.8 years and 1.5 years, respectively, for the years ended December 31, 2012 and 2011. The aggregate intrinsic value of the options exercised during the years ended December 31, 2012 and 2011 was $1 and $108, respectively.
At December 31, 2012 and 2011, 677,041 and 67,131 options, respectively, were available for grant.
F-24
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
12. Income Taxes
The Company’s financial statements indicate a total tax expense of $0 on a net loss of $11,477 and $10,206 for the years ended December 31, 2012 and 2011, respectively. A reconciliation of the difference between the benefit for income taxes and income taxes at the statutory U.S. federal income tax rate is as follows:
| Year Ended December 31, | ||||||||||||||||
| 2012 | 2011 | |||||||||||||||
| Amount | Percent of Pretax Income |
Amount | Percent of Pretax Income |
|||||||||||||
| Income taxes at statutory rate |
$ | (3,902 | ) | 34.0 | % | $ | (3,470 | ) | 34.0 | % | ||||||
| State income taxes |
(409 | ) | 3.6 | % | (370 | ) | 3.6 | % | ||||||||
| Deemed contribution interest |
718 | (6.3 | )% | 718 | (7.0 | )% | ||||||||||
| Provision to return adjustments |
(388 | ) | 3.4 | % | 22 | (0.2 | )% | |||||||||
| Permanent differences |
102 | (0.9 | )% | (207 | ) | 2.0 | % | |||||||||
| Other |
96 | (0.8 | )% | (15 | ) | 0.1 | % | |||||||||
| Increase in valuation allowance |
3,783 | (33.0 | )% | 3,322 | (32.5 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | — | — | $ | — | — | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The components of deferred tax assets and liabilities are as follows:
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Current deferred tax assets (liabilities): |
||||||||
| Accrued expenses |
$ | 908 | $ | 772 | ||||
| Stock-based compensation |
244 | 174 | ||||||
| Other |
126 | 203 | ||||||
| Accrued professional fees |
(9 | ) | (9 | ) | ||||
|
|
|
|
|
|||||
| 1,269 | 1,140 | |||||||
|
|
|
|
|
|||||
| Noncurrent deferred tax assets (liabilities) |
||||||||
| Net operating loss carryforwards |
25,182 | 21,533 | ||||||
| Capital loss carryforwards |
1,713 | 1,713 | ||||||
| Research and development credits |
2,228 | 2,228 | ||||||
| Depreciation |
1,156 | 1,080 | ||||||
| Derivative liability |
(79 | ) | (8 | ) | ||||
|
|
|
|
|
|||||
| 30,200 | 26,546 | |||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
31,469 | 27,686 | ||||||
| Valuation allowance |
(31,469 | ) | (27,686 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax asset |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
F-25
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
As of December 31, 2012 and 2011, the Company had federal net operating loss (NOL) carryforwards of approximately $64,804 and $56,383, respectively, North Carolina net economic loss (NEL) carryforwards of approximately $69,204 and $60,616, respectively, and Pennsylvania NOL carryforwards of approximately $80 and $80, respectively. The federal NOL, North Carolina NEL, and Pennsylvania NOL carryforwards begin to expire in 2020, 2015, and 2022, respectively. At December 31, 2012, the Company had federal research and development credit carryforwards of $2,095 and North Carolina credit carryforwards of $84, which begin to expire in 2020 and 2015, respectively.
At December 31, 2012 and 2011, the Company has concluded that it is more likely than not that the Company will not realize the benefit of its deferred tax assets due to its history of losses. Accordingly, the net deferred tax assets have been fully reserved.
In accordance with Section 382 of the Internal Revenue Code of 1986, as amended, a change in equity ownership of greater than 50% within a three-year period results in an annual limitation on the Company’s ability to utilize its NOL carryforwards created during the tax periods prior to the change in ownership. The Company has determined that ownership changes have occurred and as a result, a portion of the Company’s NOL carryforwards are limited.
The Company’s U.S. federal and state income tax returns are subject to examination by the tax authorities for all open tax years, 2009 forward.
The Company adopted FASB Accounting Standards Codification 740-10-25-5, Income Taxes, formerly FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes, as amended, on January 1, 2009. The difference between the tax benefit recognized in the financial statements and the tax benefit claimed in the tax return is referred to as an unrecognized tax benefit. The Company has no such unrecognized tax benefits as of December 31, 2012.
13. Net Loss per Share
Diluted loss per share is the same as basic loss per share for all periods presented because the effects of potentially dilutive items were anti-dilutive given the Company’s net loss. The following securities, presented on a common stock equivalent basis, have been excluded from the calculation of weighted average common shares outstanding because the effect is anti-dilutive:
| Year Ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| Convertible preferred stock: |
||||||||
| Series A preferred |
125,628 | 125,628 | ||||||
| Series B preferred |
1,908,797 | 2,847,948 | ||||||
| Series C preferred |
11,305,296 | 11,870,712 | ||||||
| Series C-1 preferred |
— | 984,615 | ||||||
| Series C-2 preferred |
2,447,159 | 2,347,826 | ||||||
| Warrants to purchase Series C-1 preferred stock |
200,885 | 196,923 | ||||||
| Warrants to purchase common stock |
543,027 | 267,380 | ||||||
| Stock options |
3,149,271 | 3,765,866 | ||||||
| Convertible notes |
2,833,566 | 1,282,071 | ||||||
F-26
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
Pro Forma Net Loss Per Share (unaudited)
Under the two-class method, for periods with net income, basic net income per common share is computed by dividing the net income attributable to common stockholders by the weighted average number of shares of common stock outstanding during the period. Net income attributable to common stockholders is computed by subtracting from net income the portion of current year earnings that the participating securities would have been entitled to receive pursuant to their dividend rights had all of the year’s earnings been distributed. No such adjustment to earnings is made during periods with a net loss, as the holders of the participating securities have no obligation to fund losses. Diluted net loss per common share is computed under the two-class method by using the weighted average number of shares of common stock outstanding, plus, for periods with net income attributable to common stockholders, the potential dilutive effects of stock options and warrants. In addition, the Company analyzes the potential dilutive effect of the outstanding participating securities under the “if-converted” method when calculating diluted earnings per share, in which it is assumed that the outstanding participating securities convert into common stock at the beginning of the period. The Company reports the more dilutive of the approaches (two-class or “if-converted”) as its diluted net income per share during the period. Due to net losses for the years ended December 31, 2012 and 2011, basic and diluted loss per share were the same, as the effect of potentially dilutive securities would have been anti-dilutive.
The numerator and denominator used in computing pro forma net loss per share for the year ended December 31, 2012 have been adjusted to assume the conversion of all outstanding shares of convertible preferred stock to common stock and exercise of common stock warrants issued with the convertible notes as of the beginning of the year or at the time of issuance, if later.
| Year Ended December 31, 2012 |
||||
| Numerator: |
||||
| Historical net loss |
$ | (a) | ||
| Plus: add back other expense (income) related to fair value adjustment of common stock warrants |
(b) | |||
|
|
|
|||
| Pro forma numerator for basic and diluted net loss per share |
$ | |||
|
|
|
|||
| Denominator: |
||||
| Historical denominator for basic and diluted net loss per share — weighted-average shares |
(c) | |||
| Plus: conversion of convertible preferred stock to common stock |
(d) | |||
| Plus: exercise of common stock warrants issued with the convertible notes |
(e) | |||
|
|
|
|||
| Pro forma denominator for basic and diluted net loss per share |
||||
|
|
|
|||
| Pro forma basic and diluted net loss per share |
$ | |||
|
|
|
|||
| (a) | Represents actual net loss as reported in the accompanying Statements of operations for the period presented. |
F-27
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
| (b) | Represents adjustment to remove other expense (income) related to the fair value adjustment of the long-term derivative liability for common stock warrants that are assumed to be exercised as of January 1, 2012. |
| (c) | Represents actual weighted average common shares outstanding — basic, as reported in the accompanying Statements of operations for the period presented. |
| (d) | Assumes the number of common shares that would have been outstanding had all outstanding shares of the Company’s convertible preferred stock converted into shares of common stock as of the later of the issuance dates of the convertible preferred stock or the beginning of the period presented, computed on a weighted average basis. |
| (e) | Assumes the number of common shares that would have been outstanding had the outstanding common stock warrants issued with the Company’s convertible notes been exercised as of the later of the issuance date or the beginning of the period presented. |
14. Related-Party Transactions
The Company had transactions with related parties for the years ended December 31, 2012 and 2011, as follows:
| Year Ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| Revenue |
$ | 7,424 | $ | 13,618 | ||||
| Travel expense |
77 | 62 | ||||||
Sanofi owns 100% of a subsidiary that is a customer of SCYNEXIS. Both Sanofi and the subsidiary have an investment in the Company. The Company’s related-party revenue with the subsidiary comprised 44% and 51% of total revenue as of December 31, 2012 and 2011, respectively.
15. Employee Benefit Plan
The Company has a 401(k) retirement plan, which covers all U.S. employees scheduled for and working more than 20 hours per week. The Company may provide a discretionary match with a maximum amount of 50% of the first 6% of eligible participant’s compensation, which vests ratably over four years. Contributions under the plan during 2012 and 2011 were approximately $250 and $279, respectively.
16. Gain on Sale of Asset
On May 17, 2012, the Company sold the rights to its HEOS software to a third party for consideration of $4,500. The Company received $3,500 on May 17, 2012 and recorded a gain on sale of asset of $3,412 within total operating expenses, net of transaction expenses. The Company anticipates that the remaining $1,000, which is being held by the buyer, will be received once certain contractual conditions were met. The Company did not recognize any amounts related to the $1,000 within its 2012 balance sheet or statement of operations as the contractual conditions were not met as of December 31, 2012.
F-28
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
17. Fair Value Measurements
The carrying amounts of certain financial instruments, including cash and cash equivalents, accounts receivable, unbilled services, accounts payable and accrued expenses approximate their respective fair values due to the short-term nature of such instruments.
As of December 31, 2012, the Company estimated that the fair value of its obligation under the 2010 Credit Facility was $14,485. As of December 31, 2012, the carrying value of the Company’s obligations under the Note and Warrant Purchase Agreement approximated fair value because they were callable on that date. As of December 31, 2011, the Company estimated that the fair value of its obligations under the 2010 Credit Facility was $12,447. As of December 31, 2011, the carrying value of obligations under the Note and Warrant Purchase Agreement approximated fair value since they were issuable in December 2011. The fair value of debt falls within Level 3 of the fair value hierarchy as it is significantly driven by the creditworthiness of the Company, which is an unobservable input.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The Company evaluates its financial assets and liabilities subject to fair value measurements on a recurring basis to determine the appropriate level in which to classify them for each reporting period. This determination requires significant judgments to be made. The following table summarizes the conclusions reached as of December 31, 2012 and 2011:
| Balance as of December 31, 2012 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
|||||||||||||
| Derivative liability — Series C-1 warrants |
$ | 158 | $ | — | $ | — | $ | 158 | ||||||||
| Derivative liability — Common stock warrants |
525 | $ | 525 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
$ | 683 | $ | — | $ | — | $ | 683 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2011 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
|||||||||||||
| Derivative liability — Series C-1 warrants |
$ | 236 | $ | — | $ | — | $ | 236 | ||||||||
| Derivative liability — Common stock warrants |
304 | $ | 304 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
$ | 540 | $ | — | $ | — | $ | 540 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The Company’s derivative liabilities are the only balance sheet amounts that are measured at fair value on a recurring basis. The fair value of these warrant derivatives is based on a valuation of the Company’s common stock. In order to determine the fair value of the Company’s common stock, the Company used a probability-weighted expected return method, or PWERM. Significant inputs for the PWERM included an
F-29
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
estimate of the Company’s equity value, a weighted average cost of capital and an estimated probability and timing for each valuation scenario.
A reconciliation of the beginning and ending balances for assets and liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) is as follows:
| Year Ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| Beginning balance |
$ | 540 | $ | 256 | ||||
| Issuance of warrants |
328 | 304 | ||||||
| Adjustment to fair value |
(185 | ) | (20 | ) | ||||
|
|
|
|
|
|||||
| Ending balance |
$ | 683 | $ | 540 | ||||
|
|
|
|
|
|||||
18. Restatement
Subsequent to the issuance of the Company’s 2012 financial statements, the Company’s management determined that the items discussed below were misstated. As a result, the previously reported financial information as of and for the years ended December 31, 2012 and 2011 has been restated to correct for these errors:
| Ÿ | In April 2010, the Company secured a $15,000 credit facility guaranteed by a related party that has an investment in the Company (see Note 6). The Company has concluded that a deemed contribution in relation to the guarantee should have been recognized as deferred financing costs and amortized over the 36 month life of the credit facility. The value of the guarantee was determined based on the difference between the credit facility’s stated interest rate and the interest rate that would apply had there been no guarantee from the related party. As a result, the Company has determined the value of the guarantee to be $6,338 as of April 2010. The deferred financing costs related to the guarantee are being amortized over the original life of the credit facility. The Company recorded $2,112 of amortization expense for each of the two years in the period ended December 31, 2012 related to the guarantee. The deemed contribution’s unamortized balance of deferred financing costs was $528 and $2,641 at December 31, 2012 and 2011, respectively. The balance of $528 will be amortized to expense in 2013.1 |
| Ÿ | In July 2006, the Company entered into a venture loan and security agreement (the Agreement) comprising four notes totaling $10,000. The Company also issued warrants to purchase 196,923 shares of Series C-1 Convertible Preferred Stock in conjunction with the Agreement. The warrants issued have an exercise price of $3.25 per share and will expire on July 14, 2016. The fair value of these warrants on the date of issuance was $459, and the Company recorded a debt discount and additional paid-in capital. The Company has determined that the warrants should have been recorded as a derivative liability and stated at fair value in the accompanying balance sheets. The debt discount was fully amortized as of December 31, 2010. At December 31, 2012, the warrants remained outstanding. As a result, the Company classified these warrants as a derivative liability and recorded other income of $79 and $20 for the years ended December 31, 2012 and 2011, respectively, related to the fair value adjustment of the derivative liability. The value of these warrants’ derivative liability was $158 and $236 at December 31, 2012 and 2011, respectively.2 |
| Ÿ | On August 7, 2012, the Company entered into a license agreement with a customer and received a non-refundable upfront fee of approximately $313. The Company determined that the license met |
F-30
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
| the criteria to be considered a separate unit of accounting and initially recognized the entire amount as license revenue during the year ended December 31, 2012. However, the Company has now concluded that the license fee should have been deferred and recognized on a straight-line basis over the period the services are being performed as stipulated in the license agreement. As a result, revenue was reduced by $62 for the year ended December 31, 2012.3 |
| Ÿ | In May 2012, the Company sold an asset and reported the proceeds, net of expenses, as a gain on the sale of asset in loss from continuing operations in the statement of operations. The Company determined that the gain was incorrectly presented as an operating cash flow in the statement of cash flows. As a result, the Company’s statement of cash flows should have included an adjustment to reconcile net loss to net cash used in operating activities and a cash inflow in investing activities for the proceeds from the sale of the asset.4 |
| Ÿ | The Company inappropriately recorded $90 of research and development expense in the year ended December 31, 2011. These expenses should have been recorded in the year ended December 31, 2012.5 |
Stockholders’ deficit as of January 1, 2011 was restated by $6,338 for the deemed contribution from the guarantee, $1,585 to record amortization of the deferred financing costs, and $256 to reflect the classification of warrants as a derivative liability. See the Statements of Changes in Stockholders’ Deficit. The following table details the impact of the restatement on the Company’s financial statements as of and for the years ended December 31, 2012 and 2011:
| 2012 | 2011 | |||||||||||||||||||||||
| As Reported | Adjustment | As Restated | As Reported | Adjustment | As Restated | |||||||||||||||||||
| Balance Sheets |
||||||||||||||||||||||||
| Accounts receivable, net of allowance for bad debts |
1,661 | — | 1,661 | 5 | 1,571 | 45 | 1,616 | 5 | ||||||||||||||||
| Total current assets |
5,224 | — | 5,224 | 6,195 | 45 | 6,240 | ||||||||||||||||||
| Deferred financing costs |
2 | 528 | 530 | 1 | 194 | 2,641 | 2,835 | 1 | ||||||||||||||||
| Total assets |
11,590 | 528 | 12,118 | 13,899 | 2,686 | 16,585 | ||||||||||||||||||
| Accrued expenses |
811 | — | 811 | 5 | 1,607 | (45 | ) | 1,562 | 5 | |||||||||||||||
| Deferred revenue |
120 | 62 | 182 | 3 | 225 | — | 225 | 3 | ||||||||||||||||
| Total current liabilities |
14,169 | 62 | 14,231 | 7,751 | (45 | ) | 7,706 | |||||||||||||||||
| Derivative liability |
525 | 158 | 683 | 2 | 304 | 236 | 540 | 2 | ||||||||||||||||
| Total liabilities |
31,227 | 220 | 31,447 | 24,614 | 191 | 24,805 | ||||||||||||||||||
| Additional paid-in capital |
11,312 | 6,082 | 17,394 | 3,547 | 6,082 | 9,629 | ||||||||||||||||||
| Accumulated deficit |
(77,042 | ) | (5,774 | ) | (82,816 | ) | (67,751 | ) | (3,588 | ) | (71,339 | ) | ||||||||||||
| Total stockholders’ deficit |
(65,723 | ) | 308 | (65,415 | ) | (64,200 | ) | 2,494 | (61,706 | ) | ||||||||||||||
| Statements of Operations |
||||||||||||||||||||||||
| Revenue |
9,475 | (62 | ) | 9,413 | 3 | 12,836 | — | 12,836 | 3 | |||||||||||||||
| Gross profit |
2,535 | (62 | ) | 2,473 | 3 | 8,701 | — | 8,701 | 3 | |||||||||||||||
| Research and development |
8,837 | 90 | 8,927 | 5 | 11,723 | (90 | ) | 11,633 | 5 | |||||||||||||||
| Loss from operations |
(7,632 | ) | (152 | ) | (7,784 | ) | (8,002 | ) | 90 | (7,912 | ) | |||||||||||||
| Derivative fair value adjustment |
106 | 79 | 185 | 2 | — | 20 | 20 | 2 | ||||||||||||||||
| Amortization of deferred financing costs and debt discount |
(806 | ) | (2,112 | ) | (2,918 | )1 | (26 | ) | (2,112 | ) | (2,138 | )1 | ||||||||||||
| Total other expense |
(1,660 | ) | (2,033 | ) | (3,693 | ) | (202 | ) | (2,092 | ) | (2,294 | ) | ||||||||||||
| Net loss |
(9,292 | ) | (2,185 | ) | (11,477 | ) | (8,204 | ) | (2,002 | ) | (10,206 | ) | ||||||||||||
F-31
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
| 2012 | 2011 | |||||||||||||||||||||||
| As Reported | Adjustment | As Restated | As Reported | Adjustment | As Restated | |||||||||||||||||||
| Statements of Cash Flows |
||||||||||||||||||||||||
| Gain on sales of asset, net of transaction costs |
— | (3,412 | ) | (3,412 | )4 | — | — | — | 4 | |||||||||||||||
| Amortization of deferred financing costs and debt discount |
806 | 2,112 | 2,918 | 1 | 26 | 2,112 | 2,138 | 1 | ||||||||||||||||
| Change in fair value of derivative liability |
(106 | ) | (79 | ) | (185 | )2 | — | (20 | ) | (20 | )2 | |||||||||||||
| Change in accounts receivable and unbilled services |
(475 | ) | 45 | (430 | )5 | (870 | ) | (45 | ) | (915 | )5 | |||||||||||||
| Change in accounts payable and accrued expenses |
(453 | ) | 45 | (408 | )5 | (1,261 | ) | (45 | ) | (1,306 | )5 | |||||||||||||
| Change in deferred revenue |
(105 | ) | 62 | (43 | )3 | — | — | — | 3 | |||||||||||||||
| Net cash used in operating activities |
(7,184 | ) | (3,412 | ) | (10,596 | ) | (8,958 | ) | — | (8,958 | ) | |||||||||||||
| Proceeds from sale of asset, net of transaction costs |
— | 3,412 | 3,412 | 4 | — | — | — | 4 | ||||||||||||||||
| Net cash provided by (used in) investing activities |
(361 | ) | 3,412 | 3,051 | (276 | ) | — | (276 | ) | |||||||||||||||
19. Subsequent Events
The Company evaluated subsequent events through May 24, 2013, the date on which the December 31, 2012 financial statements were originally issued, and December 20, 2013, the date on which the retrospectively revised December 31, 2012 financial statements were issued (as to the restatement described in Note 18).
There are no significant events that require disclosure in these consolidated financial statements, except as follows:
Amendment of Credit Agreement
On March 8, 2013, the Company entered into an agreement to amend the $15,000 credit facility with HSBC Bank (the “2013 Credit Agreement”) under the same terms as the 2010 Credit Agreement (see Note 6). The 2013 Credit Agreement requires interest-only payments through December 2014. All outstanding borrowings under the agreement are due on December 31, 2014. The 2013 Credit Agreement is guaranteed by a related party that has an investment in the Company. The 2013 Credit Agreement contains no financial covenants.
Issuance of convertible notes payable
On June 28, 2013, the Company entered into a Note Purchase Agreement (“the Purchase Agreement”) with certain existing lenders (“the Note Holders”). Under the Purchase Agreement, the Note Holders agreed to loan to the Company up to $1,500 in exchange for convertible notes (“the Notes”). The Company issued Notes for an aggregate amount of $899. The Notes accrue interest at 8% per annum, and the principal and all accrued and unpaid interest is due and payable on December 31, 2013. The Notes include conversion of
F-32
SCYNEXIS, INC.
NOTES TO THE FINANCIAL STATEMENTS — (Continued)
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2012 AND 2011
(in thousands, except percentage, share and per share data)
the entire outstanding principal and interest balance into equity securities upon the closing of any equity financing at the option of the Note Holders.
Also under the Purchase Agreement, the Company agreed to issue warrants to purchase shares of the Company’s common stock (the Warrants) upon the request of the majority of the Note Holders. On December 11, 2013, in connection with the stock purchase disclosed below, the Note Holders elected to receive and the Company issued Warrants to purchase 1,815,385 shares of the Company’s common stock at $0.01 per share. In addition, the Note Holders elected to convert the outstanding principal balance of the Notes and accrued interest into Series D-2 Preferred at a conversion price of $1.40 per share.
Litigation settlement
On October 9, 2013, the Company agreed to settle a claim filed against it by a former client for $195. The settlement was covered by the Company’s intellectual property insurance provider and releases the Company from any further claims or demands.
Sale of Stock and Conversion of Notes
On December 11, 2013, the Company entered into an agreement to sell 1,785,712 shares of Series D-2 Preferred at $1.40 per share for an aggregate price of $2,500 (Series D-2 Purchase Agreement). The Series D-2 Purchase Agreement also includes warrants to purchase 1,785,712 shares of the Company’s common stock at $0.01 per share.
Concurrent with the sale of the Series D-2 Preferred, the Company modified the terms of the convertible notes and warrants issued under the December 2011 Note and Warrant Purchase Agreement (Note 6). Under the amendment, the outstanding principal and accrued interest balance is convertible into Series D-1 and Series D-2 Preferred at a conversion price of $1.40 per share, and upon approval of the amendment, holders of the convertible notes elected to convert their outstanding balances.
Significant Agreements
In May 2013, the Company entered into a licensing agreement with Merck under which the Company received all human health rights for SCY-078, including all related technical documents, preclinical data, data from the seven Phase 1 clinical trials conducted by Merck, and drug product and drug substance. Merck also transferred additional quantities of active pharmaceutical ingredient, which the Company believes will be sufficient to support the development and manufacture of an IV formulation for clinical studies and provide material for additional toxicology studies. As consideration, Merck is eligible to receive milestone payments from the Company upon initiation of Phase 2 and 3 clinical trials, NDA filing and marketing approvals in each of the US, major European markets, and Japan that could total $19,000. In addition, Merck is eligible to receive tiered royalties from the Company based on a percentage of worldwide net sales of SCY-078. The aggregate royalties are mid- to high- single-digit percentages.
In August 2013, the Company entered into a development, license and supply agreement with R-Pharm, CJSC granting it exclusive rights to develop and commercialize SCY-078 in the field of human health in Russia and certain smaller non-core markets. The Company received an upfront payment and is entitled to receive payments on development milestones, commercialization targets based upon the cumulative net sales of the product, and low double-digit percentage royalties on SCY-078 sales.
F-33
SCYNEXIS, Inc.
(unaudited)
(in thousands, except share and per share data)
| September 30, 2013 |
December 31, 2012 |
September 30, 2013 |
||||||||||
| (Pro Forma) | ||||||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
$ | 926 | $ | 2,385 | $ | |||||||
| Accounts receivable, net of allowance for bad debts |
1,682 | 1,661 | ||||||||||
| Unbilled services |
409 | 757 | ||||||||||
| Prepaid expenses and other current assets |
728 | 421 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
3,745 | 5,224 | ||||||||||
| Property and equipment, net of accumulated depreciation |
5,642 | 6,284 | ||||||||||
| Deferred financing costs |
2,679 | 530 | ||||||||||
| Other assets and deferred costs |
80 | 80 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
$ | 12,146 | $ | 12,118 | $ | |||||||
|
|
|
|
|
|
|
|||||||
| Liabilities and stockholders’ deficit |
||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 854 | $ | 1,018 | $ | |||||||
| Accrued expenses |
1,155 | 811 | ||||||||||
| Deferred revenue |
1,762 | 182 | ||||||||||
| Interest payable — related party |
1,479 | 776 | ||||||||||
| Convertible notes — related party, net of discount |
11,897 | 11,444 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
17,147 | 14,231 | ||||||||||
| Long-term debt |
15,000 | 15,000 | ||||||||||
| Derivative liability |
2,522 | 683 | ||||||||||
| Deferred rent |
1,496 | 1,533 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities |
36,165 | 31,447 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Commitments and contingencies (Note 4) |
||||||||||||
| Series A convertible preferred stock, $0.001 par value, authorized 31,410 shares; 31,407 shares issued and outstanding as of September 30, 2013 and December 31, 2012; 0 shares issued and outstanding pro forma. |
250 | 250 | — | |||||||||
| Series B convertible preferred stock, $0.001 par value, authorized 711,987 shares; 467,814 shares issued and outstanding as of September 30, 2013 and December 31, 2012; 0 shares issued and outstanding pro forma. |
4,215 | 4,215 | — | |||||||||
| Series C convertible preferred stock, $0.001 par value, authorized 2,967,678 shares; 2,770,633 shares issued and outstanding as of September 30, 2013 and December 31, 2012; 0 shares issued and outstanding pro forma. |
28,121 | 28,121 | — | |||||||||
| Series C-1 convertible preferred stock, $0.001 par value, authorized 3,076,923 shares; 0 shares issued and outstanding as of September 30, 2013, December 31, 2012, and pro forma. |
— | — | — | |||||||||
| Series C-2 convertible preferred stock, $0.001 par value, authorized 2,347,826 shares; 2,347,826 shares issued and outstanding as of September 30, 2013 and December 31, 2012; 0 shares issued and outstanding pro forma. |
13,500 | 13,500 | — | |||||||||
| Series D-1 convertible preferred stock, $0.001 par value, authorized 5,000,000 shares; 0 shares issued and outstanding as of September 30, 2013, December 31, 2012, and pro forma. |
— | — | — | |||||||||
| Series D-2 convertible preferred stock, $0.001 par value, authorized 5,000,000 shares, 0 shares issued and outstanding as of September 30, 2013, December 31, 2012, and pro forma. |
— | — | — | |||||||||
| Stockholders’ deficit: |
||||||||||||
| Common stock, $0.001 par value, authorized 54,000,000 shares; 6,855,149 and 6,851,149 shares issued and outstanding as of September 30, 2013 and December 31, 2012, respectively; shares issued and outstanding pro forma. |
7 | 7 | ||||||||||
| Additional paid-in capital | 21,436 | 17,394 | ||||||||||
| Accumulated deficit | (91,548 | ) | (82,816 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ deficit |
(70,105 | ) | (65,415 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities, convertible preferred stock and stockholders’ deficit |
$ | 12,146 | $ | 12,118 | $ | |||||||
|
|
|
|
|
|
|
|||||||
See accompanying notes to financial statements.
F-34
SCYNEXIS, Inc.
CONDENSED STATEMENTS OF OPERATIONS
(unaudited)
(in thousands, except share and per share data)
| Nine Months
Ended September 30, |
||||||||
| 2013 | 2012 | |||||||
| Revenue—related party |
$ | 5,466 | $ | 5,603 | ||||
| Revenue |
7,718 | 6,385 | ||||||
|
|
|
|
|
|||||
| Total revenue |
13,184 | 11,988 | ||||||
| Cost of revenue |
12,531 | 10,690 | ||||||
|
|
|
|
|
|||||
| Gross profit |
653 | 1,298 | ||||||
| Operating expenses: |
||||||||
| Research and development |
3,203 | 6,977 | ||||||
| Selling, general and administrative |
3,150 | 3,742 | ||||||
| Gain on sale of an asset |
(988 | ) | (3,412 | ) | ||||
|
|
|
|
|
|||||
| Total operating expenses |
5,365 | 7,307 | ||||||
|
|
|
|
|
|||||
| Loss from operations |
(4,712 | ) | (6,009 | ) | ||||
| Other (expense) income: |
||||||||
| Amortization of deferred financing costs and debt discount |
(2,504 | ) | (2,141 | ) | ||||
| Interest expense — related party |
(703 | ) | (516 | ) | ||||
| Interest expense |
(142 | ) | (172 | ) | ||||
| Derivative fair value adjustment |
(671 | ) | 330 | |||||
| Other expense |
— | (8 | ) | |||||
|
|
|
|
|
|||||
| Total other expense |
(4,020 | ) | (2,507 | ) | ||||
|
|
|
|
|
|||||
| Net loss |
$ | (8,732 | ) | $ | (8,516 | ) | ||
|
|
|
|
|
|||||
| Net loss per share: |
||||||||
| Basic and diluted |
$ | (1.27 | ) | $ | (1.30 | ) | ||
| Basic and diluted, pro forma |
$ | |||||||
| Weighted-average common shares outstanding: |
||||||||
| Basic and diluted |
6,852,981 | 6,573,329 | ||||||
| Basic and diluted, pro forma |
||||||||
See accompanying notes to financial statements.
F-35
SCYNEXIS, Inc.
CONDENSED STATEMENT OF CHANGES IN CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT
(unaudited)
(in thousands, except share data)
| Series A Convertible Preferred Stock |
Series B Convertible Preferred Stock |
Series C Convertible Preferred Stock |
Series C-2 Convertible Preferred Stock |
Common Stock | Additional Paid-in Capital |
Accumulated Deficit |
Total Stockholders’ Deficit |
|||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount |
|
|
|
||||||||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2012 |
31,407 | $ | 250 | 467,814 | $ | 4,215 | 2,770,633 | $ | 28,121 | 2,347,826 | $ | 13,500 | 6,851,149 | $ | 7 | $ | 17,394 | $ | (82,816 | ) | $ | (65,415 | ) | |||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (8,732 | ) | (8,732 | ) | ||||||||||||||||||||||||||||||||||||||||||||
| Deemed contribution — debt guarantee |
— | — | — | — | — | 3,930 | — | 3,930 | ||||||||||||||||||||||||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | 4,000 | — | 3 | — | 3 | |||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | 109 | — | 109 | ||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
| Balance, September 30, 2013 |
31,407 | $ | 250 | 467,814 | $ | 4,215 | 2,770,633 | $ | 28,121 | 2,347,826 | $ | 13,500 | 6,855,149 | $ | 7 | $ | 21,436 | $ | (91,548 | ) | $ | (70,105 | ) | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
See accompanying notes to financial statements
F-36
SCYNEXIS, Inc.
CONDENSED STATEMENTS OF CASH FLOWS
(unaudited)
(in thousands)
| Nine months Ended September 30, |
||||||||
| 2013 | 2012 | |||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (8,732 | ) | $ | (8,516 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Gain on sale of asset, net of transaction expenses |
(988 | ) | (3,412 | ) | ||||
| Depreciation |
1,012 | 1,150 | ||||||
| Stock-based compensation expense |
109 | 159 | ||||||
| Amortization of deferred financing costs and debt discount |
2,504 | 2,141 | ||||||
| Allowance for bad debts |
(10 | ) | (160 | ) | ||||
| Change in fair value of derivative liability |
671 | (330 | ) | |||||
| Change in deferred rent |
(38 | ) | (18 | ) | ||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable, net and unbilled services |
337 | (798 | ) | |||||
| Prepaid expenses, other assets and deferred costs |
(307 | ) | (84 | ) | ||||
| Accounts payable and accrued liabilities |
180 | 27 | ||||||
| Interest payable — related party |
703 | 516 | ||||||
| Deferred revenue |
1,580 | 669 | ||||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(2,979 | ) | (8,656 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from investing activities: |
||||||||
| Proceeds from sale of asset, net of transaction expenses |
988 | 3,412 | ||||||
| Purchase of property and equipment |
(370 | ) | (272 | ) | ||||
|
|
|
|
|
|||||
| Net cash provided by investing activities |
618 | 3,140 | ||||||
|
|
|
|
|
|||||
| Cash flows from financing activities: |
||||||||
| Proceeds from exercise of stock options |
3 | 3 | ||||||
| Proceeds from issuance of convertible notes |
899 | 5,947 | ||||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
902 | 5,950 | ||||||
|
|
|
|
|
|||||
| (Decrease) increase in cash and cash equivalents |
(1,459 | ) | 434 | |||||
| Cash and cash equivalents, beginning of period |
2,385 | 3,976 | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents, end of period |
$ | 926 | $ | 4,410 | ||||
|
|
|
|
|
|||||
| Supplemental cash flow information: |
||||||||
| Cash paid for interest |
148 | 183 | ||||||
| Noncash financing activities: |
||||||||
| Issuance of warrants allocated to debt discount |
1,168 | 328 | ||||||
| Deemed contribution of a loan guarantee |
3,930 | — | ||||||
| Conversion of preferred shares to common shares |
— | 7,400 | ||||||
See accompanying notes to the financial statements
F-37
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
1. Description of Business and Basis of Presentation
Organization
SCYNEXIS, Inc. (“SCYNEXIS” or the “Company”) is a Delaware corporation formed on November 4, 1999. SCYNEXIS is a chemistry-focused drug discovery and development company headquartered in Research Triangle Park, North Carolina.
The Company offers its services and partnerships in the drug discovery and development phases, primarily in the form of integrated research teams consisting of medicinal, computational, analytical, and process scientists working on a collaborative basis with its customers on research projects.
Going Concern
The Company has experienced recurring losses from operations and negative cash flows due to its ongoing research and development investment in cyclophillin inhibitor and anti-fungal products. The Company also has negative working capital and debt that will become due in December 2014. The conditions described above raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The Company believes it will receive continued support from its existing investors, and it intends to raise additional funds through an initial public equity offering, the proceeds from which would enable the Company to carry on its activities and meet its obligations for at least the next 12 months. If continued support from the Company’s investors is not received or if the planned initial public offering is not successful, the Company will be required to obtain additional sources of financing through a debt or equity offering, or through the sale of assets in order to meet its obligations when they become due.
2. Summary of Significant Accounting Policies
Unaudited Interim Condensed Financial Information
The accompanying unaudited condensed financial statements and footnotes have been prepared in accordance with US GAAP as contained in the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (the “Codification” or “ASC”) for interim financial information. In the opinion of management, the interim financial information includes all adjustments of a normal recurring nature necessary for a fair presentation of the results of operations, financial position, changes in convertible preferred stock and stockholders’ deficit and cash flows. The results of operations for the nine months ended September 30, 2013 are not necessarily indicative of the results for the full year or the results for any future periods. These financial statements should be read in conjunction with the audited financial statements and related footnotes for the year ended December 31, 2012 appearing elsewhere in this prospectus.
Unaudited Pro Forma Presentation
The Company has filed a Registration Statement on Form S-1 with the U.S. Securities and Exchange Commission (the “SEC”) for the proposed initial public offering (“IPO”) of shares of its common stock. The preferred stockholders of the Company intend to consent to an automatic conversion of their preferred
F-38
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
stock into common stock if the IPO is consummated. In addition, the Company issued and committed to issue certain common stock warrants at a nominal exercise price that will expire if not exercised before the IPO. Upon exercise of these warrants, a derivative liability of $ as of September 30, 2013 will be reclassified to reduce stockholders’ deficit.
The unaudited pro forma net loss per share for the nine months ended September 30, 2013 assumes the conversion as of January 1, 2013 or the time of issuance, if later, of all outstanding shares of convertible preferred stock and the exercise of all convertible note-related common stock warrants issued or committed to be issued into an aggregate of approximately million shares of common stock upon the completion of an IPO.
The Company believes that the unaudited pro forma information is material to investors because the conversion of the convertible preferred stock into common stock and the exercise of convertible note-related common stock warrants issued or committed to be issued are expected to occur upon the closing of an IPO and, therefore, the disclosure provides a measure of total liabilities, stockholders’ deficit, and net loss per share that is comparable to what will be reported as a public company.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires the Company to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates include the accounts receivable allowance, valuation of the related party deemed contribution, the fair value of the Company’s common stock used to measure stock-based compensation for options granted to employees and nonemployees and the fair value of the Company’s derivative liability.
Accounts Receivable and Unbilled Services
Accounts receivable and unbilled services consist of amounts billed and unbilled under the Company’s service contracts with its customers. The Company extends credit to customers without requiring collateral. Accounts receivable are stated at net realizable value. On a periodic basis, the Company evaluates its accounts receivable and establishes an allowance based on its history of collections and write-offs and the current status of all receivables. The Company does not accrue interest on trade receivables. The allowance for bad debts was $241 and $251 as of September 30, 2013 and December 31, 2012, respectively.
Revenue Recognition and Deferred Revenue
The Company derives the majority of its revenue from providing contract research and development services under fee for service arrangements. The Company also has entered into collaboration arrangements in exchange for non-refundable upfront payments and consideration as services are performed. These arrangements include multiple elements such as the sale of licenses and the provision of services. Under these arrangements, the Company also is entitled to receive milestone payments and royalties in the form of a designated percentage of product sales.
F-39
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
Revenue is recognized when all of the following conditions are met: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) fees are fixed or determinable, and (iv) collection of fees is reasonably assured.
When entering into an arrangement, the Company first determines whether the arrangement includes multiple deliverables and is subject to accounting guidance in ASC subtopic 605-25, Multiple-Element Arrangements. If the Company determines that an arrangement includes multiple elements, it determines whether the arrangement should be divided into separate units of accounting and how the arrangement consideration should be measured and allocated among the separate units of accounting.
An element qualifies as a separate unit of accounting when the delivered element has standalone value to the customer. The Company’s arrangements do not include a general right of return relative to delivered elements. Any delivered elements that do not qualify as separate units of accounting are combined with other undelivered elements within the arrangement as a single unit of accounting. If the arrangement constitutes a single combined unit of accounting, the Company determines the revenue recognition method for the combined unit of accounting and recognizes the revenue over the period from inception through the date the last deliverable within the single unit of accounting is delivered.
The Company’s contract research and development services revenue is recognized in the period in which the services are performed. The Company historically has recognized milestone payments received on a straight-line basis over the remaining service period. No milestone payments were received in either period presented in the accompanying statements of operations. In arrangements that include license rights and other non-contingent deliverables, these deliverables do not have standalone value. As such, the Company accounts for the license and the non-contingent deliverables as a single combined unit of accounting. Therefore, license revenue in the form of non-refundable upfront payments is deferred and recognized over the applicable relationship period, which historically has been the estimated period of the Company’s substantive performance obligations or the period the rights granted are in effect. The Company recognizes contingent event-based payments under license agreements when the payments are received. The Company recognized an immaterial amount of license revenue from the receipt of upfront payments in the accompanying statement of operations. The Company has not received any royalty payments to date.
Amounts received prior to satisfying all revenue recognition criteria are recorded as deferred revenue in the accompanying balance sheets.
In August 2013, the Company entered into a development, license and supply agreement with R-Pharm, CJSC, granting it exclusive rights to develop and commercialize SCY-078 in the field of human health in Russia and certain smaller non-core markets. The Company received an upfront payment, which composes the substantial majority of its deferred revenue balance as of September 30, 2013, and is entitled to receive payments on contingent events, including 1) a development milestone payment upon the achievement of specified milestones; 2) sales-based payments upon R-Pharm’s achievement of specified targets for cumulative net sales of SCY-078; and 3) low double-digit percentage royalties on SCY-078 net sales.
The Company deferred the upfront payment received and is recognizing it over the estimated relationship period of 70 months, which includes the product development period and an additional period during which the Company is required to participate in a product development committee. The development
F-40
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
milestone payment is considered substantive and will be recognized when R-Pharm achieves certain specified milestones.
The sales-based payments are not considered substantive and will not be recognized until the Company 1) receives the payments, and 2) has no continuing performance obligations. If the Company has any continuing performance obligations when the sales-based payments are received, those payments will be deferred and recognized over the remaining period of continuing performance obligations. Royalties will be recognized when payment is received.
Fair Value of Financial Instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date, based on the Company’s principal or, in absence of a principal, most advantageous market for the specific asset or liability.
The Company uses a three-tier fair value hierarchy to classify and disclose all assets and liabilities measured at fair value on a recurring basis, as well as assets and liabilities measured at fair value on a non-recurring basis, in periods subsequent to their initial measurement. The hierarchy requires the Company to use observable inputs when available, and to minimize the use of unobservable inputs when determining fair value. The three tiers are defined as follows:
| Ÿ | Level 1 — Observable inputs that reflect quoted market prices (unadjusted) for identical assets or liabilities in active markets; |
| Ÿ | Level 2 — Observable inputs other than quoted prices in active markets that are observable either directly or indirectly in the marketplace for identical or similar assets and liabilities; and |
| Ÿ | Level 3 — Unobservable inputs that are supported by little or no market data, which require the Company to develop its own assumptions about the assumptions market participants would use in pricing the asset or liability based on the best information available in the circumstances. |
Comprehensive Loss
The Company has no items of comprehensive income or loss other than net loss.
Stock-Based Compensation
The Company measures and recognizes compensation expense for all stock-based payment awards made to employees, officers, and directors based on the estimated fair values of the awards as of grant date. The value of the portion of the award that is ultimately expected to vest is recorded as expense over the requisite service periods.
The Company also accounts for equity instruments issued to non-employees using a fair value approach. The Company values equity instruments, stock options and warrants granted to lenders and consultants using the Black-Scholes valuation model. The measurement of non-employee share-based compensation is subject to periodic adjustments as the underlying equity instruments vest and is recognized as an expense over the term of the related financing or the period over which services are received.
F-41
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
Basic and Diluted Net Loss per Share of Common Stock
The Company uses the two-class method to compute net loss per common share because the Company has issued securities, other than common stock, that contractually entitle the holders to participate in dividends and earnings of the Company. The two-class method requires earnings for the period to be allocated between common stock and participating securities based upon their respective rights to receive distributed and undistributed earnings. Holders of each series of the Company’s convertible preferred stock are entitled to participate in distributions, when and if declared by the board of directors that are made to common stockholders, and as a result are considered participating securities.
Segment and Geographic Information
Operating segments are defined as components of an enterprise (business activity from which it earns revenue and incurs expenses) about which discrete financial information is available and regularly reviewed by the chief operating decision maker in deciding how to allocate resources and in assessing performance. The Company’s chief operating decision maker (“CODM”) is the Chief Executive Officer. The CODM reviews consolidated operating results to make decisions about allocating resources and assessing performance for the entire Company. The Company views its operations and manages its business as one operating segment. All assets of the Company were held in the United States for the nine months ended September 30, 2013.
Although all operations are based in the United States, the Company generated a portion of its revenue from customers outside of the United States. Information about the Company’s revenue from different geographic regions for the nine months ended September 30, 2013 and 2012 is presented as follows:
| For the nine months ended September 30, | ||||||||||||||||
| 2013 | 2012 | |||||||||||||||
| United States |
$ | 11,961 | 91% | $ | 9,371 | 78% | ||||||||||
| Europe |
1,223 | 9% | 2,617 | 22% | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 13,184 | 100% | $ | 11,988 | 100% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
All sales, including sales outside of the United States, are denominated in the United States Dollar.
3. Debt Obligations
Credit Facility Agreement
In April 2010, the Company entered into a $15,000 credit facility agreement with HSBC bank (the “2010 Credit Agreement”). The agreement comprises a $5,000 term loan and a $10,000 revolving credit facility. Borrowings under the 2010 Credit Agreement carry interest at a rate of London InterBank Offered Rate plus 0.95% per annum. The weighted-average interest rate was 1.2% and 1.4% for the nine months ended September 30, 2013 and the year ended December 31, 2012, respectively. As of September 30, 2013 and December 31, 2012, both the $5,000 term loan and the $10,000 revolving credit facility were outstanding. The 2010 Credit Agreement required interest-only payments through March 2013. All outstanding borrowings under the agreement were due on March 11, 2013. The 2010 Credit Agreement is guaranteed by a related party that has an investment in the Company. The 2010 Credit Agreement contains no financial covenants.
F-42
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
On March 8, 2013, the Company entered into an agreement to amend the 2010 Credit Agreement with HSBC Bank (the “2013 Credit Agreement”). The 2013 Credit Agreement requires interest-only payments through December 2014. All outstanding borrowings under the agreement are due on December 31, 2014. Other significant terms of the 2010 Credit Agreement remained the same. The 2013 Credit Agreement is guaranteed by a related party that has an investment in the Company.
At the inception of the 2010 Credit Agreement, a deemed contribution in relation to the guarantee of the 2010 Credit Agreement was recognized as deferred financing costs and amortized over the life of the loan. The value of the guarantee was determined based on the difference between the loan’s stated interest rate and the interest rate that would apply if there had been no guarantee from the related party. The Company determined the value of the 2010 Credit Agreement guarantee to be $6,338 which was being amortized over the original life of the loan. The Company determined that the 2013 Credit Agreement represented a new loan. Therefore, the value of the extended guarantee of the 2013 Credit Agreement of $3,930 is being amortized over the term of the loan.
Note and Warrant Purchase Agreements
In December 2011, the Company executed a Note and Warrant Purchase Agreement (“December 2011 Note and Warrant Agreement”) for an aggregate amount not to exceed $15,000. In 2011 and 2012, the Company issued convertible notes (“2011-2012 Notes”) with a total principal amount of $11,444 to related parties that hold investments in the Company. The 2011-2012 Notes included warrants to purchase 530,719 shares of the Company’s common stock at $0.01 per share.
The 2011-2012 Notes are convertible into shares of the Company’s stock through different methods, including:
| Ÿ | In the event the Company issues and sells shares of its equity securities to investors on or before June 30, 2012, in an equity financing with total proceeds actually received by the Company of not less than $25,000 including the conversion of the aggregate principal amount and all unpaid accrued interest outstanding under the convertible notes (a “Qualified Financing”), the outstanding principal balance of the convertible notes shall automatically convert in whole without any further action by the noteholders into such equity securities at a price equal to 85% of the issue price of such equity securities. Equity securities shall mean any series of preferred stock that (i) ranks pari passu or senior to the Company’s Series C-2 Convertible Preferred Stock upon any liquidation, dissolution or winding-up of the Company and upon any acquisition or asset transfer and (ii) is convertible into shares of common stock of the Company. This conversion option is no longer available given it expired on June 30, 2012. |
| Ÿ | Upon the occurrence of either an acquisition or asset transfer, the entire outstanding principal balance of the convertible notes shall at the option of the noteholder either (i) become fully due and payable, provided however that the repayment shall also require prior written consent of the noteholder majority and HSBC Bank or (ii) convert into whole shares of the Company’s Series D-1 or Series D-2 Preferred Stock as applicable at a conversion price equal to $4.3125 per share subject to proportionate and equitable adjustment upon any stock split, stock dividend, reverse stock split or other similar event. |
F-43
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
| Ÿ | Upon closing by the Company of any equity financing that is not a Qualified Financing, the entire principal balance of the convertible notes and all unpaid accrued interest shall at the sole option of the noteholder convert in whole into the same class or type of equity securities sold by the Company in connection with such equity financing. The conversion price shall be at a conversion price that is equal to the price paid by the investors participating in such equity financing and shall otherwise be on the same terms and conditions applicable to such investors. |
| Ÿ | Upon written consent of the Company and noteholder majority, the aggregate principal balance of the convertible notes and all accrued interest shall be automatically converted into shares of the Company’s Series D-1 Preferred stock or Series D-2 Preferred stock as applicable pursuant to the conversion price detailed above at any time on or after December 31, 2012. |
None of the events that trigger conversion of the 2011-2012 Notes occurred in the nine-month period ended September 30, 2013.
In June 2013, the Company executed another Note and Warrant Purchase Agreement (“June 2013 Note and Warrant Agreement”) with certain existing lenders. Under the June 2013 Note and Warrant Agreement, the lenders agreed to loan to the Company up to $1,500 in exchange for convertible notes (“June 2013 Notes”). The Company issued June 2013 Notes for an aggregate amount of $899. Also under the June 2013 Note and Warrant Agreement, the Company agreed to issue warrants to purchase shares of the Company’s common stock upon the request of the majority of the note holders. The warrants were issued in December 2013, as further described in Note 11. The June 2013 Notes are convertible into shares of the Company’s stock through the same methods as described above for the 2011-2012 Notes. In addition, the June 2013 Notes include conversion of the entire outstanding principal and interest balance into equity securities upon the closing of any equity financing at the option of the note holders. Events that could trigger conversion of the June 2013 Notes have not occurred in the nine-month period ended September 30, 2013.
The 2011-2012 Notes and June 2013 Notes bear interest at a rate of 8% per annum and contain no financial covenants. The outstanding principal amount and unpaid accrued interest on the convertible notes issued under the December 2011 Note and Warrant Agreement and the June 2013 Note and Warrant Agreement are due on December 31, 2012 and December 31, 2013, respectively, contingent upon (i) the prior written consent of holders of at least 70% of the outstanding aggregated principal amount of the convertible notes issued under the same agreement, and (ii) the prior written consent of HSBC Bank for so long as any of the principal and interest related to the 2010 Credit Agreement remains outstanding. These events did not occur as of September 30, 2013, and thus, the convertible notes were outstanding as of September 30, 2013.
As described in Note 11 in December 2013, the holders of the 2011-2012 Notes and the June 2013 Notes elected to convert the outstanding principal and accrued interest under the Notes into Series D-1 Preferred and Series D-2 Preferred.
Total notes payable due as of September 30, 2013 and December 31, 2012 were classified as current and amounted to $11,897 and $11,444, net of discount of $446 and $0, respectively.
The Company accounted for an embedded put option in the June 2013 Notes under the derivative accounting guidance. Under this guidance, a company may be required to bifurcate an embedded feature from its host instrument and account for the embedded derivative as a free-standing derivative financial
F-44
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
instrument that is measured at fair value at issuance and adjusted to its current fair value at each period. The Company determined that the put option should be bifurcated from the June 2013 Notes and recorded at fair value. The fair value of the embedded put option was nil at issuance and September 30, 2013.
On the date of issuance, the fair value of warrants issued in the nine months ended September 30, 2012 was $328. The fair value of issued warrants was accounted for as debt discount and amortized to expense over the stated term of the 2011-2012 Notes. The fair value of the obligation to issue warrants in connection with the June 2013 Notes was $1,168. The fair value of the obligation to issue warrants was $269 above the face value of the June 2013 Notes and this excess was expensed at issuance. The $899 remaining amount of the fair value of the obligation to issue warrants was accounted for as a debt discount and is being amortized to expense over the term of the June 2013 Notes. The amount of the discount related to the 2011-2012 Notes’ warrants and the June 2013 Notes’ obligation to issue warrants that was amortized to expense for the nine-months ended September 30, 2013 and 2012 was $709 and $412, respectively.
Future Debt Maturities
Future debt maturities as of September 30, 2013 are as follows:
| 2013 |
$ | 12,343 | ||
| 2014 |
15,000 | |||
|
|
|
|||
| $ | 27,343 | |||
|
|
|
4. Commitments and Contingencies
Leases
The Company leases its facilities and certain office equipment under long-term non-cancelable operating leases. The Company’s lease for its primary North Carolina facility expires in 2014. The lease has two optional five-year renewal periods through 2024. The first optional renewal period for the original lease space has been included in the future minimum lease payments, as the Company would incur a significant economic penalty through relocation or replacement of leasehold improvements prior to the end of their useful lives.
Rent expense was $687 and $668 for the nine months ended September 30, 2013 and 2012, respectively. Future minimum lease payments for all operating expenses as of September 30, 2013 are as follows:
| 2013 |
$ | 231 | ||
| 2014 |
1,049 | |||
| 2015 |
1,104 | |||
| 2016 |
1,137 | |||
| 2017 |
1,171 | |||
| Thereafter |
1,510 | |||
|
|
|
|||
| Total |
$ | 6,202 | ||
|
|
|
F-45
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
License Arrangement with Potential Future Expenditures
As of September 30, 2013, the Company had a license arrangement with Merck that involves potential future expenditures. Under the terms of the license agreement, Merck is eligible to receive milestone payments from the Company upon initiation of phase 2 and 3 clinical studies, NDA filing and marketing approvals in each of the US, major European markets and Japan that could total $19,000. In addition, Merck is eligible to receive tiered royalties from the Company based on a percentage of worldwide net sales of SCY-078. The aggregate royalties are mid- to high-single digits. The Company has two additional licensing agreements that could require it to make payments of up to $2,500 upon achievement of certain milestones by the Company.
5. Convertible Preferred Stock
Convertible Preferred stock has par value $0.001 and was issued beginning in 2000. Each issuance is briefly described as follows:
Series A Convertible Preferred Stock (“Series A Preferred”)
In 2000, the Company issued 31,407 shares of Series A Preferred at $7.96 per share to initial employees and consultants of SCYNEXIS.
Series B Convertible Preferred Stock (“Series B Preferred”)
In 2000, the Company issued 600,999 shares of Series B Preferred at $9.01 per share in exchange for $2,200 in equipment, intellectual property, and conversion of existing debt, and $3,215 in cash and incurred issuance costs of $43. Subsequently in 2000, the Company issued an additional 110,988 shares of Series B convertible preferred stock at $9.01 per share for cash. As part of the issuance of the Series C convertible preferred stock in June 2002, the holders of Series B Preferred agreed to modify the redemption feature of the Series B Preferred to eliminate the redemption feature of the Series B Preferred. 244,173 shares of Series B Preferred were mandatorily converted to common stock during 2012.
Series C Convertible Preferred Stock (“Series C Preferred”) and Warrants
The Company issued warrants to purchase 100,524 shares of Series C Preferred in conjunction with certain bridge loan financings during 2001 and the subsequent 2002 Series C Preferred financing. The warrants were issued with an exercise price of $0.01 per share. Two of the investors exercised such warrants during 2003.
In 2002, the Company issued 2,867,154 shares of Series C Preferred for $24,000 in cash and the conversion of approximately $4,513 of 4.5% convertible notes and accrued interest, less issuance costs of approximately $86. 197,045 shares of Series C Preferred were mandatorily converted to common stock during 2012. In January 2005, the remaining warrants to purchase 23,911 shares of Series C Preferred shares were fully exercised.
Series C-1 Convertible Preferred Stock (“Series C-1 Preferred”) and Warrants
In August 2004, the Company received cash of $3,200 for the issuance of 984,615 shares of Series C-1 Preferred. These Series C-1 Preferred shares were mandatorily converted to common stock during 2012.
In July 2006, the Company issued warrants to purchase 196,923 shares of Series C-1 Preferred in conjunction with a loan financing agreement. The warrants were issued with an exercise price of $3.25 per
F-46
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
share and expire on July 14, 2016. The fair value at the date of grant for these instruments was $459, which was recorded as a debt discount. The debt discount related to these warrants was fully amortized as of December 31, 2010. The Company determined that the warrants should be recorded as a derivative liability and stated at fair value at each reporting period.
Series C-2 Convertible Preferred Stock (“Series C-2 Preferred”)
In March 2008, the Company received cash of $13,500 for the issuance of 2,347,826 shares of Series C-2 Preferred.
Series D-1 Convertible Preferred Stock (“Series D-1 Preferred) and Series D-2 Convertible Preferred Stock (“Series D-2 Preferred”)
Shares of Series D-1 Preferred and Series D-2 Preferred (together “Series D Preferred”) are authorized, but none are issued or outstanding as of September 30, 2013 and December 31, 2012.
Redemption
Upon liquidation, dissolution, or winding up of the Company, the holders of the Series D-2 Preferred receive an amount equal to three times the original issue price plus all declared and unpaid dividends; the holders of the Series D-1 Preferred receive an amount equal to two times the original issue price plus all declared and unpaid dividends; and the holders of the Series C-2 Preferred, Series C-1 Preferred, Series C Preferred, Series B Preferred, and the Series A Preferred receive an amount equal to the original issue price plus all declared and unpaid dividends. In addition, after receiving their liquidation preference, the holders of all series of preferred stock share ratably with holders of common stock on an as-if-converted to common stock basis. An asset transfer or acquisition of the Company is a deemed liquidation event in that holders of all series of preferred stock are treated in the same manner as upon liquidation, dissolution, or winding up of the Company. As a result of the existence of this deemed liquidation feature, the Company determined that all series of preferred stock are redeemable. They are carried at liquidation value at each reporting period and excluded from stockholders’ deficit in the accompanying balance sheets.
F-47
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
Authorized, Issued and Outstanding Preferred Shares
There were no issuances of Series A Preferred, Series B Preferred, Series C Preferred, Series C-1 Preferred, Series C-2 Preferred, Series D-1 Preferred, and Series D-2 Preferred for the nine months ended September 30, 2013. The following table summarizes authorized, issued and outstanding preferred shares as of September 30, 2013:
| Authorized | Outstanding | Issue Price | Liquidation Preference |
|||||||||||||
| Series A Preferred |
31,410 | 31,407 | $ | 7.96 | $ | 250 | ||||||||||
| Series B Preferred |
711,987 | 467,814 | 9.01 | 4,215 | ||||||||||||
| Series C Preferred |
2,967,678 | 2,770,633 | 10.15 | 28,121 | ||||||||||||
| Series C-1 Preferred |
3,076,923 | — | 3.25 | — | ||||||||||||
| Series C-2 Preferred |
2,347,826 | 2,347,826 | 5.75 | 13,500 | ||||||||||||
| Series D-1 Preferred |
5,000,000 | — | 4.31 | — | ||||||||||||
| Series D-2 Preferred |
5,000,000 | — | 4.31 | — | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total |
19,135,824 | 5,617,680 | $ | 46,086 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
There were no changes in significant terms of the convertible preferred stock during the nine months ended September 30, 2013.
6. Common Stock Warrants
In 2007, in connection with the procurement of a debt financing agreement used to purchase equipment during that year, the Company issued warrants to purchase 12,308 shares of common stock. The warrants were issued with an exercise price of $3.25 per share and will expire on September 14, 2014. The fair value at the date of grant for these instruments was insignificant.
In 2012 and 2011, in connection with the issuance of convertible notes, the Company issued warrants to purchase 530,719 shares of common stock (see Note 3 for disclosures regarding the convertible notes). The warrants may be exercised into common stock at the earliest of:
| (i) | the date the related convertible notes are converted in accordance with the terms above, |
| (ii) | the date the related convertible notes are repaid or prepaid in full in accordance with the terms above, and |
| (iii) | June 30, 2012. |
No warrants were exercised during the nine months ended September 30, 2013 or 2012. These warrants will expire on June 30, 2017. The exercise price of the warrants is $0.01 per share of common stock and the number of shares of common stock that may be purchased by exercising the warrants is calculated as follows:
| Ÿ | If a related convertible note is converted pursuant to and in accordance with the terms above, then the number of shares of common stock shall be equal to the quotient of (A) the product of (i) 20% multiplied by (ii) the principal amount of the convertible note divided by (B) the applicable per share conversion price at which the related convertible note is so converted; or |
F-48
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
| Ÿ | If a related convertible note is repaid or prepaid in full prior to the conversion thereof pursuant to and in accordance with the terms above, then the number of shares of common stock shall be equal to the quotient of (A) the product of (i) 20% multiplied by (ii) the principal amount of the convertible note divided by (B) $4.3125 (subject to proportionate adjustment upon any adjustment to the Series D-1 Preferred or Series D-2 Preferred conversion price as defined in the Company’s Fourth Amended and Restated Certificate of Incorporation); or |
| Ÿ | If a warrant is first exercised at any time after June 30, 2012, and such first exercise of the warrant occurs prior to the conversion, repayment or prepayment of the related convertible note pursuant to and in accordance with the terms above, then the number of shares of common stock shall be equal to the quotient of (A) the product of (i) 20% multiplied by (ii) the principal amount of the convertible note divided by (B) $4.3125 (subject to proportionate adjustment upon any adjustment to the Series D-1 Preferred or Series D-2 Preferred conversion price as defined in the Company’s Fourth Amended and Restated Certificate of Incorporation). |
These warrants meet the definition of a derivative financial instrument and are accounted for as derivatives.
Also under the June 2013 Note and Warrant Agreement (Note 3), the Company agreed to issue warrants to purchase shares of the Company’s common stock upon the request of the majority of the note holders. The warrants were issued in December 2013, as further described in Note 11. The obligation to issue warrants meets the definition of a derivative financial instrument and is accounted for as a derivative.
The combined fair values of the common stock warrant derivative liabilities are $2,463 and $525 as of September 30, 2013 and December 31, 2012, respectively, and are recorded as a long-term derivative liability in the balance sheet. The Company recorded other expense of $770 and other income of $212 for the nine months ended September 30, 2013 and 2012, respectively, related to the fair value adjustment of the long-term derivative liability for common stock warrants.
7. Net Loss per Common Share
The following instruments, presented on a common stock equivalent basis, have been excluded from the calculation of weighted average common shares outstanding because the effect is anti-dilutive:
| Nine Months
Ended September 30, |
||||||||
| 2013 | 2012 | |||||||
| Convertible preferred stock: |
||||||||
| Series A preferred |
125,628 | 125,628 | ||||||
| Series B preferred |
1,908,797 | 1,908,797 | ||||||
| Series C preferred |
11,305,296 | 11,305,296 | ||||||
| Series C-2 preferred |
2,447,159 | 2,447,159 | ||||||
| Warrants to purchase Series C-1 preferred stock |
200,885 | 200,885 | ||||||
| Warrants to purchase common stock |
1,722,970 | 543,027 | ||||||
| Stock options |
2,969,151 | 3,150,164 | ||||||
| Convertible notes |
3,205,118 | 2,780,545 | ||||||
F-49
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
Pro Forma Net Loss Per Share
Under the two-class method, for periods with net income, basic net income per common share is computed by dividing the net income attributable to common stockholders by the weighted average number of shares of common stock outstanding during the period. Net income attributable to common stockholders is computed by subtracting from net income the portion of current year earnings that the participating securities would have been entitled to receive pursuant to their dividend rights had all of the year’s earnings been distributed. No such adjustment to earnings is made during periods with a net loss, as the holders of the participating securities have no obligation to fund losses. Diluted net loss per common share is computed under the two-class method by using the weighted average number of shares of common stock outstanding, plus, for periods with net income attributable to common stockholders, the potential dilutive effects of stock options and warrants. In addition, the Company analyzes the potential dilutive effect of the outstanding participating securities under the “if-converted” method when calculating diluted earnings per share, in which it is assumed that the outstanding participating securities convert into common stock at the beginning of the period. The Company reports the more dilutive of the approaches (two-class or “if-converted”) as its diluted net income per share during the period. Due to net losses for the nine months ended September 30, 2013 and 2012, basic and diluted loss per share were the same, as the effect of potentially dilutive securities would have been anti-dilutive.
The numerator and denominator used in computing pro forma net loss per share for the nine months ended September 30, 2013 have been adjusted to assume the conversion of all outstanding shares of convertible preferred stock to common stock and exercise of common stock warrants issued with the convertible notes as of the beginning of the year or at the time of issuance, if later.
| Nine Months Ended September 30, 2013 |
||||
| Numerator: |
||||
| Historical net loss |
$ | (a) | ||
| Plus: add back other expense (income) related to fair value adjustment of common stock warrants |
(b) | |||
|
|
|
|||
| Pro forma numerator for basic and diluted net loss per share |
$ | |||
|
|
|
|||
| Denominator: |
||||
| Historical denominator for basic and diluted net loss per share — weighted-average shares |
(c) | |||
| Plus: conversion of convertible preferred stock to common stock |
(d) | |||
| Plus: exercise of common stock warrants issued with the convertible notes |
(e) | |||
|
|
|
|||
| Pro forma denominator for basic and diluted net loss per share |
||||
|
|
|
|||
| Pro forma basic and diluted net loss per share |
$ | |||
|
|
|
|||
| (a) | Represents actual net loss as reported in the accompanying Statements of operations for the period presented. |
F-50
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
| (b) | Represents adjustment to remove other expense (income) related to the fair value adjustment of the long-term derivative liability for common stock warrants that are assumed to be exercised as of January 1, 2013. |
| (c) | Represents actual weighted average common shares outstanding — basic, as reported in the accompanying statements of operations for the period presented. |
| (d) | Assumes the number of common shares that would have been outstanding had all outstanding shares of the Company’s convertible preferred stock converted into shares of common stock as of the later of the issuance dates of the convertible preferred stock or the beginning of the period presented, computed on a weighted average basis. |
| (e) | Assumes the number of common shares that would have been outstanding had the outstanding common stock warrants issued with the Company’s convertible notes and common stock warrants that the Company is obligated to issue under the June 2013 Note and Warrant Agreement been exercised as of the later of the date the obligation to issue was made or the beginning of the period presented. |
8. Related-Party Transactions
The Company had transactions and balances with related parties as of and for the nine months ended September 30, 2013 and 2012, as follows:
| For the nine months ended September 30, |
||||||||
| 2013 | 2012 | |||||||
| Revenue |
$ | 5,466 | $ | 5,603 | ||||
| Travel Expenses |
26 | 17 | ||||||
Sanofi owns 100% of a subsidiary that is a customer of SCYNEXIS. Both Sanofi and the subsidiary have an investment in the Company. The Company’s related-party revenue with the subsidiary comprised 42% and 47% of total revenue for the nine-month periods ended September 30, 2013 and 2012, respectively.
9. Gain on Sale of Asset
On May 17, 2012, the Company sold the rights to its HEOS software to a third party for consideration of $4,500. The Company received $3,500 on May 17, 2012 and recorded a gain on sale of asset of $3,412 within total operating expenses, net of transaction expenses. The remaining balance of $1,000 was held in escrow by the buyer until certain conditions were met.
On May 17, 2013, the Company met all the contractual conditions and collected the $1,000 held in escrow. The Company recognized $988, which is net of transaction expenses, as a gain on sale of asset within total operating expenses.
10. Fair Value Measurements
The carrying amounts of certain financial instruments, including cash and cash equivalents, accounts receivable, unbilled services, accounts payable and accrued expenses approximate their respective fair values due to the short-term nature of such instruments.
F-51
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
As of September 30, 2013, the Company estimated that the fair value of its obligations under the 2013 Credit Agreement was $12,581. As of September 30, 2013, the Company estimated that the fair value of its obligations under the Note and Warrant Purchase Agreement was $12,008. The fair value of debt falls within Level 3 of the fair value hierarchy as it is significantly driven by the creditworthiness of the Company, which is an unobservable input.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The Company evaluates its financial assets and liabilities subject to fair value measurements on a recurring basis to determine the appropriate level in which to classify them for each reporting period. This determination requires significant judgments to be made. The following table summarizes the conclusions reached as of September 30, 2013 and 2012:
| Balance as of September 30, 2013 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant
Unobservable Inputs (Level 3) |
|||||||||||||
| Derivative liability — |
$ | 59 | $ | — | $ | — | $ | 59 | ||||||||
| Derivative liability — |
2,463 | $ | 2,463 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
$ | 2,522 | $ | — | $ | — | $ | 2,522 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2012 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant
Unobservable Inputs (Level 3) |
|||||||||||||
| Derivative liability — |
$ | 158 | $ | — | $ | — | $ | 158 | ||||||||
| Derivative liability — |
525 | $ | 525 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
$ | 683 | $ | — | $ | — | $ | 683 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The Company’s derivative liabilities are the only balance sheet amounts that are measured at fair value on a recurring basis. The fair value of these warrant derivatives is based on a valuation of the Company’s common stock. In order to determine the fair value of the Company’s common stock, the Company used a probability-weighted expected return method, or PWERM. Significant inputs for the PWERM included an estimate of the Company’s equity value, a weighted average cost of capital and an estimated probability and timing for each valuation scenario.
F-52
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
A reconciliation of the beginning and ending balances for assets and liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) is as follows:
| Nine months
ended September 30, |
||||||||
| 2013 | 2012 | |||||||
| Beginning balance |
$ | 683 | $ | 540 | ||||
| Issuance of warrants |
1,168 | 328 | ||||||
| Adjustment to fair value |
671 | (330 | ) | |||||
|
|
|
|
|
|||||
| Ending balance |
$ | 2,522 | $ | 538 | ||||
|
|
|
|
|
|||||
11. Subsequent Events
The Company evaluated subsequent events through December 20, 2013, the date on which the September 30, 2013 interim financial statements were originally issued. The Company also has evaluated subsequent events through January 28, 2014, the date on which the September 30, 2013 interim financial statements were available to be reissued. There are no additional significant events that require disclosure in these interim financial statements except as follows:
Issuance of common stock warrants and conversion of June 2013 notes
On December 11, 2013, in connection with the stock purchase disclosed below, the holders of notes issued under the June 2013 Note and Warrant Agreement (“Notes”) elected to receive and the Company issued warrants to purchase 1,815,385 shares of the Company’s common stock at $0.01 per share. In addition, the Note holders elected to convert the outstanding principal balance of the Notes and accrued interest into Series D-2 Preferred at a conversion price of $1.40 per share.
Litigation settlement
On October 9, 2013, the Company agreed to settle a claim filed against it by a former client for $195. The settlement was covered by the Company’s intellectual property insurance provider and releases the Company from any further claims or demands.
Sale of Stock and Conversion of Notes
On December 11, 2013, the Company entered into an agreement to sell 1,785,712 shares of Series D-2 Preferred at $1.40 per share for an aggregate price of $2,500 (Series D-2 Purchase Agreement). The Series D-2 Purchase Agreement also includes warrants to purchase 1,785,712 shares of the Company’s common stock at $0.01 per share.
Concurrent with the sale of the Series D-2 Preferred, the Company modified the terms of the convertible notes and warrants issued under the December 2011 Note and Warrant Purchase Agreement (Note 3). Under the amendment, the outstanding principal and accrued interest balance is convertible into Series D-1 and Series D-2 Preferred at a conversion price of $1.40 per share, and upon approval of the amendment, holders of the convertible notes elected to convert their outstanding balances.
F-53
SCYNEXIS, Inc.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2013
(in thousands, except percentage, share and per share data)
Lease Renewal
The Company entered into a lease renewal for its primary facility in December 2013. The lease extends through March 31, 2019 the existing lease that otherwise would expire on March 31, 2014, and includes a renewal option to extend the lease through March 31, 2024.
Licensing Agreement
The Company entered into a licensing agreement with a major animal health company in December 2013. The agreement includes an upfront payment, multi-year contract research and development services, and entitles the Company to potential milestone and single-digit percentage royalty payments on net sales of product.
F-54
Shares

SCYNEXIS, Inc.
Common Stock
PROSPECTUS
RBC CAPITAL MARKETS
, 2014
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other expenses of issuance and distribution
The following table sets forth the costs and expenses, other than underwriting discounts and commissions, payable by us in connection with the sale and distribution of our common stock being registered. All amounts are estimates except for the SEC registration fee, the FINRA filing fee, and the listing fee of the NASDAQ Global Market.
| SEC registration fee |
$ | * | ||
| FINRA filing fee |
* | |||
| NASDAQ Global Market listing fee |
* | |||
| Legal fees and expenses |
* | |||
| Accounting fees and expenses |
* | |||
| Printing and engraving expenses |
* | |||
| Transfer agent and registrar fees and expenses |
* | |||
| Blue sky fees and expenses |
* | |||
| Miscellaneous fees and expenses |
* | |||
|
|
|
|||
| Total |
$ | * | ||
|
|
|
| * | To be completed by amendment. |
Item 14. Indemnification of directors and officers
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities, including reimbursement for expenses incurred, arising under the Securities Act of 1933, as amended, or the Securities Act.
Our amended and restated certificate of incorporation that will be in effect upon the closing of this offering provides for indemnification of our directors, officers, employees and other agents to the maximum extent permitted by the Delaware General Corporation Law, and our amended and restated bylaws that will be in effect upon the closing of this offering provide for indemnification of our directors, officers, employees and other agents to the maximum extent permitted by the Delaware General Corporation Law.
We have entered and expect to continue to enter into agreements to indemnify our directors and executive officers. With certain exceptions, these agreements provide for indemnification for related expenses including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding.
We maintain insurance policies that indemnify our directors and officers against various liabilities arising under the Securities Act and the Exchange Act of 1934, as amended, that might be incurred by any director or officer in his capacity as such.
In an underwriting agreement we enter into in connection with the sale of our common stock being registered hereby, or the Underwriting Agreement, the underwriters will agree to indemnify, under certain circumstances, us, our officers, our directors, and our controlling persons within the meaning of the Securities Act, against certain liabilities.
II-1
Item 15. Recent sales of unregistered securities
The following sets forth information regarding all unregistered securities sold during the last three years:
Preferred Stock Issuances
| Ÿ | On December 11, 2013, we sold 1,785,712 shares of our Series D-2 Preferred Stock and warrants exercisable for 1,785,712 shares of our common stock to five investors for aggregate proceeds of $2.5 million, which we refer to as our 2013 financing. In addition, we issued 6,054,255 shares of Series D-1 Preferred Stock and 3,956,985 shares of Series D-2 Preferred Stock in connection with the conversion of all outstanding principal and interest on the convertible promissory notes previously issued in our 2011 bridge financing and 2013 bridge financing, described below. |
Convertible Note and Warrant Issuances
| Ÿ | On December 7, 2011, January 27, 2012 and May 15, 2012, we collectively issued and sold (i) an aggregate principal amount of $11.4 million of convertible promissory notes and (ii) warrants to purchase an aggregate of 530,719 shares of our common stock with an exercise price of $0.01 per share for aggregate proceeds of $5,722, to eleven investors, which we refer to as our 2011 bridge financing. In connection with our 2013 financing, these warrants were adjusted to be exercisable for 1,634,792 shares of our common stock with no additional proceeds to us. |
| Ÿ | On June 28, 2013, we issued and sold an aggregate principal amount of $899,053 convertible promissory notes to six investors, which we refer to as our 2013 bridge financing. |
| Ÿ | On December 11, 2013, pursuant to the terms of our 2013 bridge financing, we issued warrants exercisable for 1,815,385 shares our common stock with an exercise price of $0.01 per share to six investors with no additional proceeds to us. |
Option and Common Stock Issuances
| Ÿ | From December 1, 2010 to date, we issued pursuant to our 2009 Stock Option Plan options exercisable for an aggregate of 885,856 of our common stock, of which no options to purchase shares of our common stock have been exercised, options to purchase 121,614 shares had been forfeited and options to purchase 764,242 shares remained outstanding, at a weighted average exercise price of $1.32 per share to certain of our officers, employees, directors and consultants. |
| Ÿ | From December 1, 2010 to date, we issued an aggregate of 151,200 shares of our common stock to certain of our officers, employees, directors and consultants for an aggregate purchase price of $73,052 pursuant to the exercise of options issued under our 1999 Stock Option Plan. |
| Ÿ | On January 27, 2012, we issued an aggregate of 2,777,117 shares of our common stock to three holders of our preferred stock upon the conversion of such preferred stock into shares or our common stock with no additional proceeds to us. |
The sales of the preferred stock, warrant and convertible notes described above were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(2) of the Securities Act (or Regulation D promulgated thereunder). The sales of the options and common stock above were deemed to be exempt from registration under the Securities Act in reliance upon Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving any public offering or pursuant to benefit plans and contracts relating to compensation as provided under Rule 701. The issuance of shares of preferred stock upon conversion of outstanding convertible promissory notes were deemed to be exempt from registration in reliance on Section 3(a)(9) of the Securities Act. We did not pay or give, directly or
II-2
indirectly, any commission or other remuneration, including underwriting discounts or commissions, in connection with any of the issuances of securities listed above. The recipients of the preferred stock, warrants and convertible notes in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed upon the stock certificates issued in these transactions. All recipients had adequate access, through their employment or other relationship with us or through other access to information provided us, to information about us. The sales of these securities were made without any general solicitation or advertising.
Item 16. Exhibits and financial statement schedules
| (a) | Exhibits |
| Exhibit No. |
Description | |
| 1.1* | Form of Underwriting Agreement. | |
| 3.1** | Amended and Restated Certificate of Incorporation, as amended and as currently in effect. | |
| 3.2* | Form of Amended and Restated Certificate of Incorporation to become effective upon closing of this offering. | |
| 3.3** | Bylaws, as amended and as currently in effect. | |
| 3.4* | Form of Amended and Restated Bylaws to become effective upon closing of this offering. | |
| 4.1* | Form of Common Stock Certificate of the Registrant. | |
| 5.1* | Opinion of Cooley LLP. | |
| 10.1* | Form of Indemnity Agreement between the Registrant and its directors and officers. | |
| 10.2** | SCYNEXIS, Inc. 1999 Stock Option Plan, as amended, and Forms of Stock Option Grant Notice, Stock Option Agreement and Notice of Stock Option Exercise. | |
| 10.3** | SCYNEXIS, Inc. 2009 Stock Option Plan, as amended, and Forms of Stock Option Grant Notice, Stock Option Agreement and Notice of Stock Option Exercise. | |
| 10.4* | SCYNEXIS, Inc. 2014 Equity Incentive Plan and Form of Stock Option Agreement and Form of Stock Option Grant Notice thereunder. | |
| 10.5* | SCYNEXIS, Inc. 2014 Employee Stock Purchase Plan. | |
| 10.6* | Non-Employee Director Compensation Policy. | |
| 10.7** | Amended and Restated Employment Agreement, dated December 7, 2012, between SCYNEXIS, Inc. and Charles F. Osborne, Jr. | |
| 10.8** | Employment Agreement, dated August 20, 2012, between SCYNEXIS, Inc. and Eileen C. Pruette. | |
| 10.9** | Amended and Restated Employment Agreement, dated December 7, 2012, between SCYNEXIS, Inc. and Yves J. Ribeill. | |
| 10.10†** | Development, License and Supply Agreement, dated August 1, 2013, between SCYNEXIS, Inc. and R-Pharm, CJSC. | |
| 10.11†** | License Agreement, dated August 7, 2012, as amended, between SCYNEXIS, Inc. and Dechra Ltd. | |
II-3
| Exhibit No. |
Description | |
| 10.12†** | Termination and License Agreement, dated May 24, 2013, between SCYNEXIS. Inc. and Merck Sharp & Dohme Corp. | |
| 10.13†** | Agreement for the Assignment of Patents and Know How concerning Cyclosporin Derivatives, dated June 10, 2005, between SCYNEXIS, Inc. and C-CHEM AG. | |
| 10.14†** | Research Services Agreement, dated December 19, 2011 between SCYNEXIS, Inc. and Merial Limited. | |
| 10.15†** | Exclusive Worldwide License Agreement, dated May 10, 2005, between SCYNEXIS, Inc. and Aventis Pharma S.A. | |
| 10.16** | Amendment No. 1 to Exclusive Worldwide License Agreement, dated October 26, 2006, between SCYNEXIS, Inc. and Aventis Pharma S.A. | |
| 10.17 | Letter Agreement, dated April 9, 2010, as amended, between SCYNEXIS, Inc. and HSBC Bank USA, National Association. | |
| 10.18 | Stand Alone First Demand Guarantee, dated April 9, 2010, as amended, in favor of SCYNEXIS, Inc., by and between Sanofi-Aventis S.A. and HSBC Bank USA, National Association. | |
| 10.19 | Reimbursement & General Security Agreement, dated April 9, 2010, between SCYNEXIS, Inc. and Sanofi-Aventis S.A. | |
| 10.20 | Guarantee Extension Agreement, dated March 5, 2013, between SCYNEXIS, Inc. and Sanofi-Aventis S.A. | |
| 10.21** | Fifth Amended and Restated Investor Rights Agreement, dated December 11, 2013. | |
| 10.22** | Industrial Building Lease, dated as of July 1, 2007, as amended, between SCYNEXIS, Inc. and Durham Research Tri-Center, LLC. | |
| 10.23† | Amended and Restated License, Development and Commercialization Agreement, dated December 23, 2013, between SCYNEXIS, Inc. and Elanco Animal Health. | |
| 10.24 | Employment Agreement, dated January 2014, between SCYNEXIS, Inc. and Carole A. Sable. | |
| 10.25 | Offer Letter, dated September 16, 2013, from SCYNEXIS, Inc. to Vivian W. Doelling. | |
| 10.26 | Board Observation Rights Agreement, dated March 5, 2013, between SCYNEXIS, Inc. and Sanofi-Aventis S.A. | |
| 10.27 | Form of Lock-up Agreement between the Registrant and its directors and officers. | |
| 23.1* | Consent of Independent Registered Public Accounting Firm. | |
| 23.2* | Consent of Cooley LLP. Reference is made to Exhibit 5.1. | |
| 24.1* | Power of Attorney. | |
| * | To be filed by amendment. |
| ** | Previously filed. |
| † | Confidential Treatment Requested. |
| (b) | Financial Statement Schedules |
II-4
Item 17. Undertakings
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the Underwriting Agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (2) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Durham, State of North Carolina, on , 2014.
| SCYNEXIS, INC. | ||
| By: |
| |
| Yves J. Ribeill, Ph.D. | ||
| President and Chief Executive Officer | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitute and appoint Yves J. Ribeill, Ph.D., Eileen C. Pruette and Charles F. Osborne, Jr., and each one of them, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place, and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to sign any registration statement for the same offering covered by this registration statement that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act, and all post-effective amendments thereto, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or his, her or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
|
Yves J. Ribeill, Ph.D. |
President, Chief Executive Officer and Director (Principal Executive Officer) |
, 2014 | ||
|
Charles F. Osborne, Jr. |
Chief Financial Officer (Principal Financial and Accounting Officer) |
, 2014 | ||
|
Pamela J. Kirby, Ph.D. |
Chairman of the Board of Directors | , 2014 | ||
|
Laurent Arthaud |
Director | , 2014 | ||
|
Mounia Chaoui, Ph.D. |
Director | , 2014 | ||
|
Ann F. Hanham, Ph.D. |
Director | , 2014 | ||
II-6
| Signature |
Title |
Date | ||
|
Patrick J. Langlois, Ph.D. |
Director | , 2014 | ||
|
Jean-Yves Nothias, Ph.D. |
Director | , 2014 | ||
|
Edward E. Penhoet, Ph.D. |
Director | , 2014 | ||
II-7